» Postlab Questions Bonding and Moleculary Geometry V2 » 10-064: Mo Course Contents » 10-064: Molecu lar Bonds And Dipole Moments Consider the following molecules: A - BrF3 В - PF3 D - F2 C- BrCl E- CF4 Which one of these molecules has bonds that are most polar? B Draw Lewis stru ctures and determine the molecular shapes. You must have both answers correct. Which have a molecular dipole moment? BrF3 PF3 BrCl F2 CF4 Submit Answer Incorrect. Tries 1/5 Previous Tries This discussion is closed. Type here to search e L
» Postlab Questions Bonding and Moleculary Geometry V2 » 10-064: Mo Course Contents » 10-064: Molecu lar Bonds And Dipole Moments Consider the following molecules: A - BrF3 В - PF3 D - F2 C- BrCl E- CF4 Which one of these molecules has bonds that are most polar? B Draw Lewis stru ctures and determine the molecular shapes. You must have both answers correct. Which have a molecular dipole moment? BrF3 PF3 BrCl F2 CF4 Submit Answer Incorrect. Tries 1/5 Previous Tries This discussion is closed. Type here to search e L
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 70P: Ozone (O3) has a nonzero dipole moment. In the molecule of O3 , one of the oxygen atoms is directly...
Related questions
Question
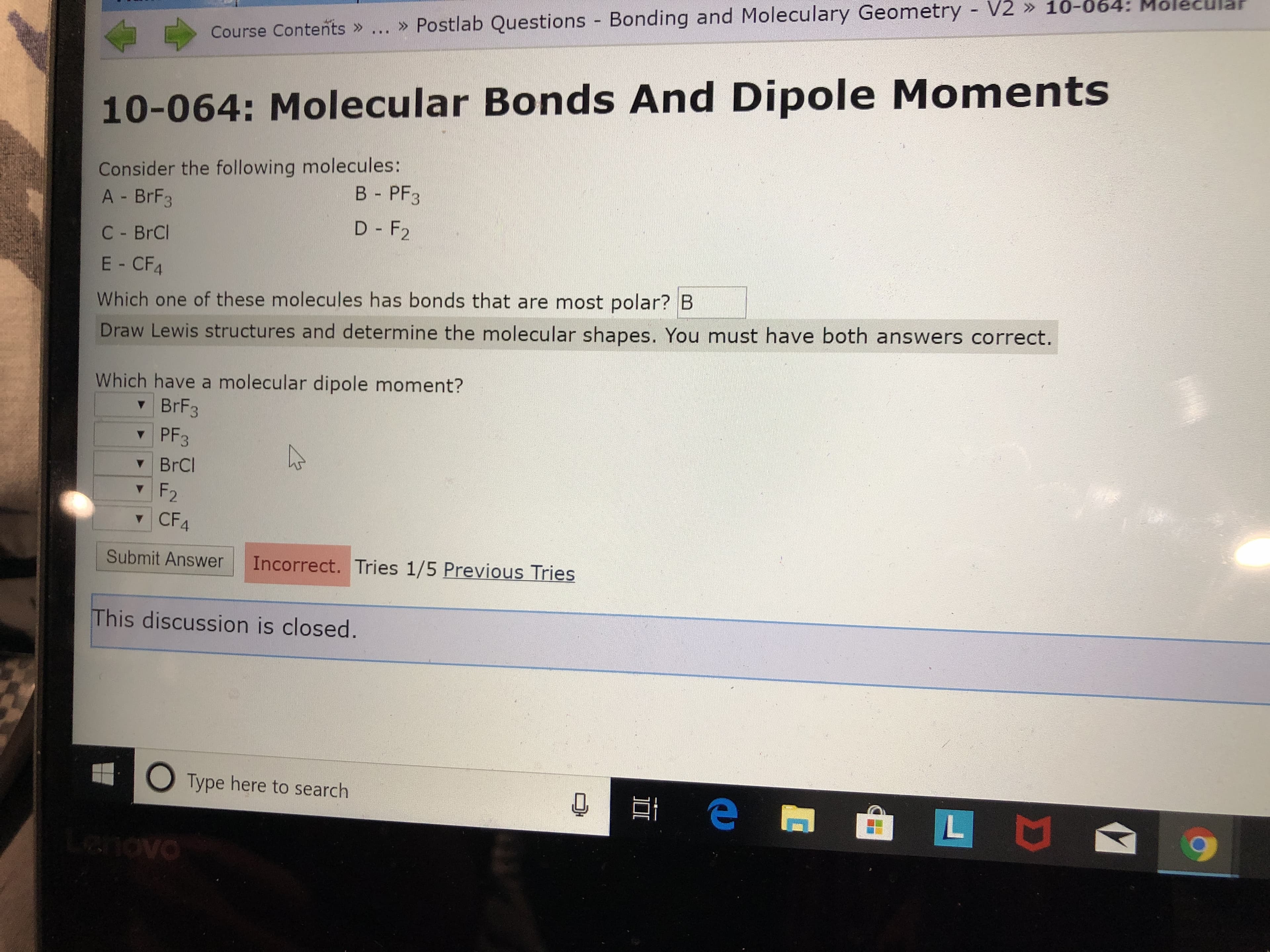
Transcribed Image Text:» Postlab Questions Bonding and Moleculary Geometry V2 » 10-064: Mo
Course Contents »
10-064: Molecu lar Bonds And Dipole Moments
Consider the following molecules:
A - BrF3
В - PF3
D - F2
C- BrCl
E- CF4
Which one of these molecules has bonds that are most polar? B
Draw Lewis stru ctures and determine the molecular shapes. You must have both answers correct.
Which have a molecular dipole moment?
BrF3
PF3
BrCl
F2
CF4
Submit Answer
Incorrect. Tries 1/5 Previous Tries
This discussion is closed.
Type here to search
e
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning