Problem 18 18 of 27 Review I Constants I Periodic Table Assuming a total pressure of 9.8 atm, what is the partial pressure of helium in the mixture of 98 % helium and 2.0 % oxygen breathed by deep-sea divers? Express your answer using two significant figures. ΑΣφ ? atm Submit Request Answer Part B What is the partial pressure of oxygen in the mixture? Express your answer using two significant figures. VA ΑΣΦ atm P Pearson Contact Us Copyright O 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy | Permissions F12 II F8 F11 F10 F9 DO0 O00 F7 F6 F5 F4 F3
Problem 18 18 of 27 Review I Constants I Periodic Table Assuming a total pressure of 9.8 atm, what is the partial pressure of helium in the mixture of 98 % helium and 2.0 % oxygen breathed by deep-sea divers? Express your answer using two significant figures. ΑΣφ ? atm Submit Request Answer Part B What is the partial pressure of oxygen in the mixture? Express your answer using two significant figures. VA ΑΣΦ atm P Pearson Contact Us Copyright O 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy | Permissions F12 II F8 F11 F10 F9 DO0 O00 F7 F6 F5 F4 F3
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.8: The Behavior Of Real (non-ideal) Gases
Problem 8.18E
Related questions
Question
Part A and B with breakdown.. Don't understand how to set this problem up
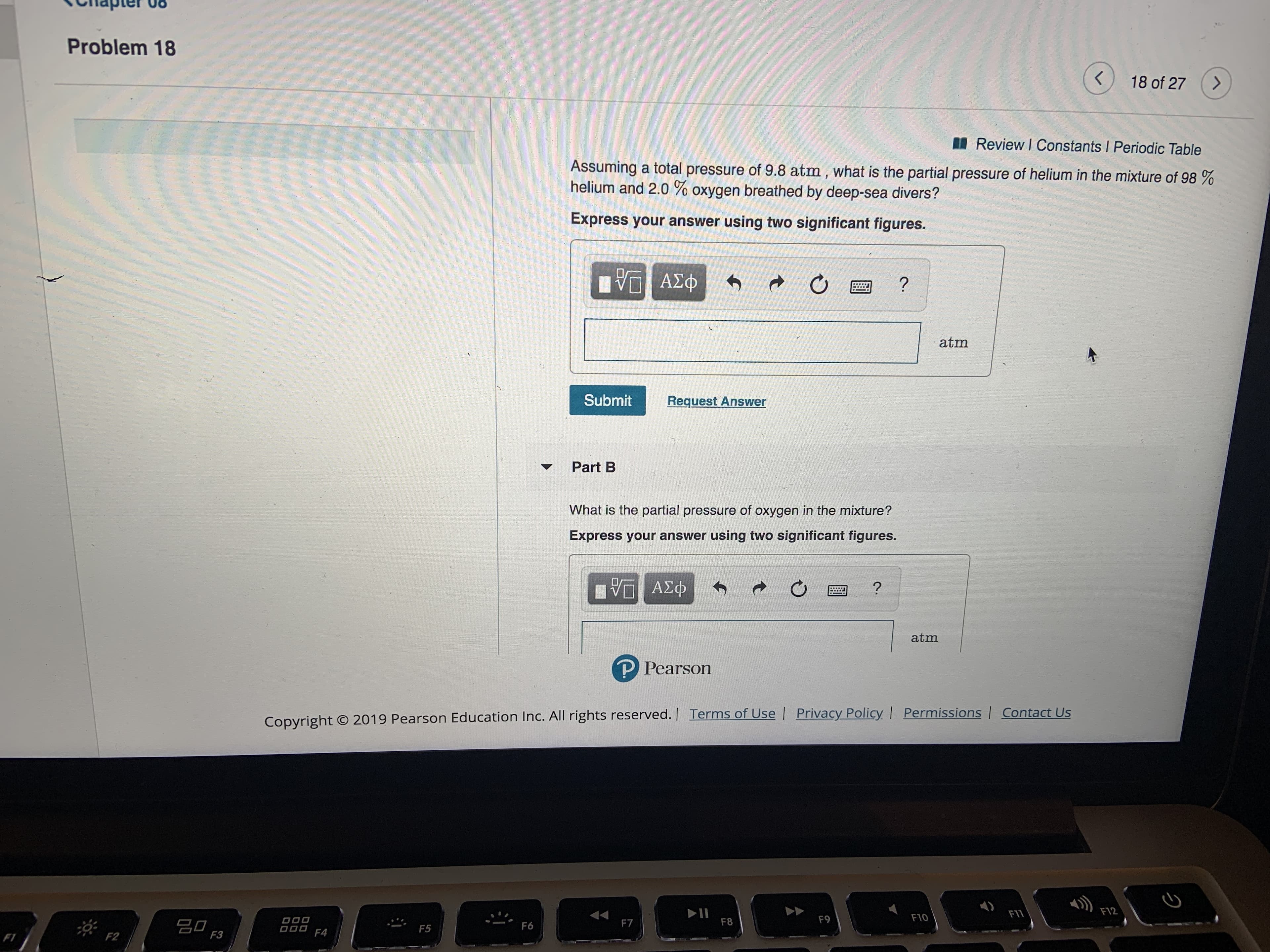
Transcribed Image Text:Problem 18
18 of 27
Review I Constants I Periodic Table
Assuming a total pressure of 9.8 atm, what is the partial pressure of helium in the mixture of 98 %
helium and 2.0 % oxygen breathed by deep-sea divers?
Express your answer using two significant figures.
ΑΣφ
?
atm
Submit
Request Answer
Part B
What is the partial pressure of oxygen in the mixture?
Express your answer using two significant figures.
VA
ΑΣΦ
atm
P Pearson
Contact Us
Copyright O 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy | Permissions
F12
II
F8
F11
F10
F9
DO0
O00
F7
F6
F5
F4
F3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning