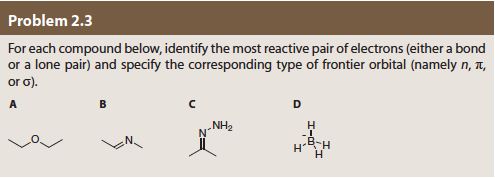
Problem 2.3 For each compound below, identify the most reactive pair of electrons (either a bond or a lone pair) and specify the corresponding type of frontier orbital (namely n, t or ơ). NH2
Q: Example 4. exocyclic double bond for ring-C and also ring-A C A в exocyclic double bond for ring-C…
A:
Q: Problem : IUPAC names? (a) CH3 (b) CH,CH,CH, (c) CH3 CH3 (d) CH,CH3 (e) CH3 (f) Br CH(CH3)2 `CH3 Br…
A: Name of Organic compounds based on IUPAC nomenclature system. This system has different rules - (1)…
Q: Below is the SN2 reaction between chlorocyclohexane and cyanide (CN-). Draw the missing curved arrow…
A: The SN2 reaction between chlorocyclohexane and cyanide (CN-) is given below,
Q: Vanillin (Figure 5, C8H8O3) is an organic compound extracted from the vanilla bean and is used to…
A: A double bond is formed with an sp2-hybridized orbital. A single bond is formed with an…
Q: Problem 3: Constitutional isomers are compounds that possess the same molecular formula but…
A: Note - Since you have posted a question with multiple sub-parts, we will solve first three…
Q: statement a: Three sp2 hybridized orbital shells are determined if a fully hbridized carbon atom has…
A:
Q: Problem 2.3 Draw structural formulas for the three constitutional isomers with the molecular formula…
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Question attached
A: While drawing the resonance structures, the lone pair electrons are converted to bond pair electrons…
Q: This question has multiple parts. Work all the parts to get the most points. a For each compound…
A:
Q: Rizatriptan (trade name Maxalt) is a prescription drug used for the treatment of migraines. (a) How…
A: (a) Hello. Since your question has multiple sub-parts, we will solve first three sub-parts for you.…
Q: Problem 3: Draw the molecular orbitals for each of the following, Be sure to label every sigma (a)…
A: The molecular orbital for formaldehyde is drawn as shown below.
Q: Q1. Provide IUPAC name for the following compounds. a) b) c) HO, "Br
A: ***Note: Since you have posted a question with multiple subparts, we will solve the first three…
Q: 6b. Convert the line-angle formula below into condensed formula: Br
A: The compounds given are,
Q: Which one of the following bonds is the most polar? Group of answer choices C-S C-H C-O C-N
A: The answer to this question is as follows--------- When two bonded atoms have different…
Q: II. Research for structure (submit on short bond paper, handwritten only) 1) Phenolphthalein -a…
A: Phenolphthalein is an acid-base indicator. Hexachlorophene used as disinfectant. Urushiol contained…
Q: 2. Draw the following bond-line structures in dash-wedge notation, showing all implied hydrogens.…
A: bond line is 2 dimensional representation where as dash wedge is 3 dimensional representation.
Q: сompound name CH — сн, — сн, CH, — сн, — с — сH, — сн, — сн, CH, — сн, — сн — сн, CH, сH, — сH, — сн…
A: According to the question, we need to determine the IUPAC name of the given hydrocarbon. First…
Q: • PRACTICE PROBLEM 5.2 Give names that include (R) and (S) designations for compounds B and C in…
A:
Q: Draw a circle the compound below with the strongest C-CI bond and draw a rectangle around the…
A: The compound is mostly stabilized by the resonance effect . More is the resonance effect , more will…
Q: Current Attempt in Progress Name these ketones: 1 H, (a) CH,ċ-CCH, CH, (b) CH,CCH,CH,CH, CH, (c)…
A:
Q: (a) Add curved arrows to show how the starting material A is converted to the product B. (b) Draw…
A: Given, the structure of A is: The curved arrows to convert A into B is shown below:
Q: Calculate the number of double bond equivalencies each formula has. Blank 1: C17H16NBr Blank 2:…
A: Double bond equivalent(DBE) is also termed as degree of unsaturation. DBE is the number of molecules…
Q: a) Draw the Lewis structure of the nitrate ion, NO3 -, including all resonance forms and formal…
A: a) Lewis structure for any molecule is drawn by using the following steps, First the skeletal…
Q: State whether the following compound is E or Z
A: Geometrical isomers are have same bond connectivity of atoms but differ in their spacial…
Q: Problem : IUРАС names? (а) CH3 (b) CH,CH,CH3 (с) CH3 CH3 (d) CH,CH3 „CH3 (f) Br CH(CH3)2 CH3 Br…
A: IUPAC stands for International Union of Pure and Applied Chemistry. Every compound has one IUPAC…
Q: Step 2: When a curved arrow starts from a lone pair and points to an atom, a new sigma bond will…
A:
Q: Draw a line angle formula of a compound that has a ring of 6 carbons and the composition C7H14
A:
Q: Answer following question about oxycodone, a narcotic analgesic used forsevere pain. Question : How…
A: Hybridization refers to the intermixing of atomic orbitals which leads to the formation of new…
Q: Which is a correct resonance structure for compound A below: H2C=CH-C*H-CH=O (Compound A) 2.…
A:
Q: Which molecule(s) will experience the Hydrogen Bridging force? Select all that apply. F2 CF4 HF H2O
A:
Q: Identify the correct statement which is related to ?aromatic hydrocarbon :Select one .a It has only…
A: The correct statement about aromatic hydrocarbon is given below
Q: PRACTICE PROBLEM 2.12 Write bond-line structural formulas for (a) two primary alcohols, (b) a…
A: In organic chemistry, different structures are represented by same Molecular formula, this property…
Q: Problem 1.41 ralla two sp³ hybridized carbons. Express your answer as a condensed structural…
A: sp3 hybridised carbons contain single bonds. sp2 hybridised carbons contain double bonds. sp…
Q: он HO
A: Given: To find: water solubility highest Melting point Van der Waal interaction dipole-dipole…
Q: Problem : IUPAC names? CH (a) The three isomers of C;H12 (b) CH CH,CHCHCH3 CH,CH3 CH3 CH3 (c)…
A: (a) Pentane has Molecular formula of C5H12 , and it has three isomers - n- pentane (pentane), iso-…
Q: Problem 1. How many sigma and pi bonds are present in this molecule of propylene? H H. H H
A: The molecule contain 8 sigma-bonds and 2 pi-bond.
Q: different structures that have the same molecular formula but different connectivities for c5h12
A: The structural formula interprets the arrangement of all bonded atoms with the help of atomic…
Q: e) Circle the most basic nitrogen atom in the compound below. NH2
A: Since you have asked multiple question, we will solve the first question for you. If youwant any…
Q: Circle the most stable molecule in each of the following pairs. a) CH, b) CH, CH, CH, CH, CH c)…
A:
Q: Considering the molecule below, in what type of orbital does each lone pair of electrons on oxygen…
A:
Q: 3) Draw as many resonance structures as you c a) The maximum the Species %| CH3-C-CH2
A: When one structure can’t explain all the properties of a compound then more than one structures are…
Q: Example 1: How many hydrogens are in C,H,N, which has 0 ring(s) and 2 double bond(s)? Example 2: How…
A:
Q: A polar compound will have a (smaller/larger) Rf when a polar stationary phase is used
A: Retention factor= distance travelled by solute/distance travelled by solvent
Q: Problem 1.12 Draw a line-bond structure for propene, CH3CH=CH2. Indicate the hybrid- ization of each…
A: For determination of hybridization and bond angle in any molecule, it is the first step to express…
Q: CH3 N. CH3 CH3
A: Structure of the given compound,
Q: Problem 2.2 Do the line-angle formulas in each of the following sets represent the same compound or…
A: Constitutional isomers are the isomers having same molecular formula but different bond…
Q: =0 A В
A: The question is based on the concept of polarity. we have to determine which of the given molecule…
Q: Select all that is true pertaining to orbitals used in bonding and bond angles. A. All the carbon…
A: Amines are tetrahedral with sp3 hybridization. But Amides are planar with sp2 hybridization because…
Q: PROBLEM SET – CARBOXYLIC ACIDS 1. Give IUPAC names for the following carboxylic acids: (a) CH3 (b)…
A:
Q: Draw bond-line structures for all constitutional isomers of the following compound: CH3CH2CH(CH3)k…
A: Given compound is pentane. Constitutional isomers ?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

- Hw problem wants 2 structures that have the same molecular formula but different connectivities for C3H4Chemistry is it the one on the right becuae the reaonance structure of the conj base is more delocalized/stable than the conj base of the left? can you draw a resonance of both so i can check my work? thanks guys10. Question shown in photo Vanillin (Figure 5, C8H8O3) is an organic compound extracted from the vanilla bean and is used to add flavor to drinks, food, and pharmaceuticals. While natural vanilla extract is composed of many different compounds, artificial vanillin is usually synthesized as a pure compound. As of 2001 only about 15% of the annual demand for vanillin was isolated as a natural product. Identify the hybridization of the labeled atoms in vanillin. a) i (sp2 ); ii (sp2 ); iii (sp2 ) b) i (sp2 ); ii (sp2 ); iii (sp3 ) c) i (sp2 ); ii (sp3 ); iii (sp2 ) d) i (sp3 ); ii (sp2 ); iii (sp2 )
- Rizatriptan (trade name Maxalt) is a prescription drug used for the treatment of migraines. (a) How many aromatic rings does rizatriptan contain? (b) Determine the hybridization of each N atom. (c) In what type of orbital does the lone pair on each N reside? (d) Draw all the resonance structures for rizatriptan that contain only neutral atoms. (e) Draw all reasonableresonance structures for the ve-membered ring that contains three N atoms.Problem 4.73 Identify bonds polar non polarI need help with this practice problem: Draw the structure for cis-1-ethyl-2-(1-methylethyl)cyclohexane. Draw both chair conformations of this molecule and place them in the correct box based on their stability.
- Below is the SN2 reaction between iodocyclohexane and cyanide (CN–). Draw the missing curved arrow notation in the first box to reflect electron movements. In both boxes, add lone pairs of electrons and nonzero formal charges.Problem (#2.) For each ion below, draw all reasonable resonance structures (linked by resonance arrows “↔”). Include curved arrows that indicate the movement of electrons between each resonance structure. Assign non-zero formal charges to each atom for each resonance structure. (a.) NO3– (nitrate) (b.) CH3COO– (acetate) (c.) N3– (azide) (d.) NCO– (isocyanate) Problem (#3.) For each ion in question 2, draw a resonance hybrid, assigning non-zero formal and/or partial charges (δ+, δ–) as needed. Problem (#4.) For each skeletal structure below, satisfy the valences (or octets) of all of the atoms by filling in double and triple bonds as well as unshared electron pairs. Assign non-zero formal charges and show the overall charge if the structure is an ion. See photo attached for Problem number 4. Problem (#5.) For each structure in question 4, draw a resonance hybrid (if it has one) and assign non-zero formal and/or partial charges as needed.statement a: Three sp2 hybridized orbital shells are determined if a fully hbridized carbon atom has a double bond. statement b: The remaining p-orbit al signifies that it is ready for bonding. a. if the first statement is true and the second is false b. if the first statemetne is false and second is true c. if both true d. if both false
- Help! a.) Draw all the important resonance contributors for the structure shown below. Use curved arrows to show the movement of electrons between these contributors. b.) Draw the hybrid resonance hybrid structure.(a) Add curved arrows to show how the starting material A is converted to the product B. (b) Draw all reasonable resonance structures for B. (c) Draw the resonance hybrid for B.Among the most common over-the-counter drugs you might find in a medicine cabinet are mild pain relievers such ibuprofen (Advil, Motrin), naproxen (Aleve), and acetaminophen (Tylenol). (a) How many sp3-hybridized carbons does each molecule have? (b) How many sp2-hybridized carbons does each molecule have? (c) Can you spot any similarities in their structures?



