Problem 4. Calcium chloride is a salt used in a number of food and medicinal applications and in fluid for refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form it efficiently absorbs water vapour from gases, and from aqueous liquid solutions it can form (under different conditions) calcium chloride hydrate (CaCl2-H20), dihydrate (CaCl2 2H2O), tetrahydrate (CaCl2-4H2O) and hexahydrate (CaCl2-6H2O). You have been given the task of determining the standard heat of of the reaction in which calcium chloride hexahydrate is formed from anhydrous calcium chloride: AH (k) ? CaCl2(s)6H20 CaCl2-6H20 (s) By definition, the desired quantity is the heat of hydration of calcium chloride hexahydrate. You cannot carry out the hydration reaction directly, so you resort to an indirect moethod. You first dissolve 1 mol of anhydrous (no water) CaCl2 in 10 mol of water in a calorimeter and determine that 64.85 kJ of heat must be transferred away from the calorimeter to keep the solution temperature at 25°C. You next dissolve 1 mol of the hexahydrate salt in 4 mol of water and find that 32.41 kJ of heat must be transferred to the calorimeter to keep the temperature at 25°C Use these results to calculate the desired heat of reaction (Hint: remember Hess' Law)
Problem 4. Calcium chloride is a salt used in a number of food and medicinal applications and in fluid for refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form it efficiently absorbs water vapour from gases, and from aqueous liquid solutions it can form (under different conditions) calcium chloride hydrate (CaCl2-H20), dihydrate (CaCl2 2H2O), tetrahydrate (CaCl2-4H2O) and hexahydrate (CaCl2-6H2O). You have been given the task of determining the standard heat of of the reaction in which calcium chloride hexahydrate is formed from anhydrous calcium chloride: AH (k) ? CaCl2(s)6H20 CaCl2-6H20 (s) By definition, the desired quantity is the heat of hydration of calcium chloride hexahydrate. You cannot carry out the hydration reaction directly, so you resort to an indirect moethod. You first dissolve 1 mol of anhydrous (no water) CaCl2 in 10 mol of water in a calorimeter and determine that 64.85 kJ of heat must be transferred away from the calorimeter to keep the solution temperature at 25°C. You next dissolve 1 mol of the hexahydrate salt in 4 mol of water and find that 32.41 kJ of heat must be transferred to the calorimeter to keep the temperature at 25°C Use these results to calculate the desired heat of reaction (Hint: remember Hess' Law)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter5: Alkenes: Bonding, Nomenclature, And Properties
Section: Chapter Questions
Problem 5.31P
Related questions
Question
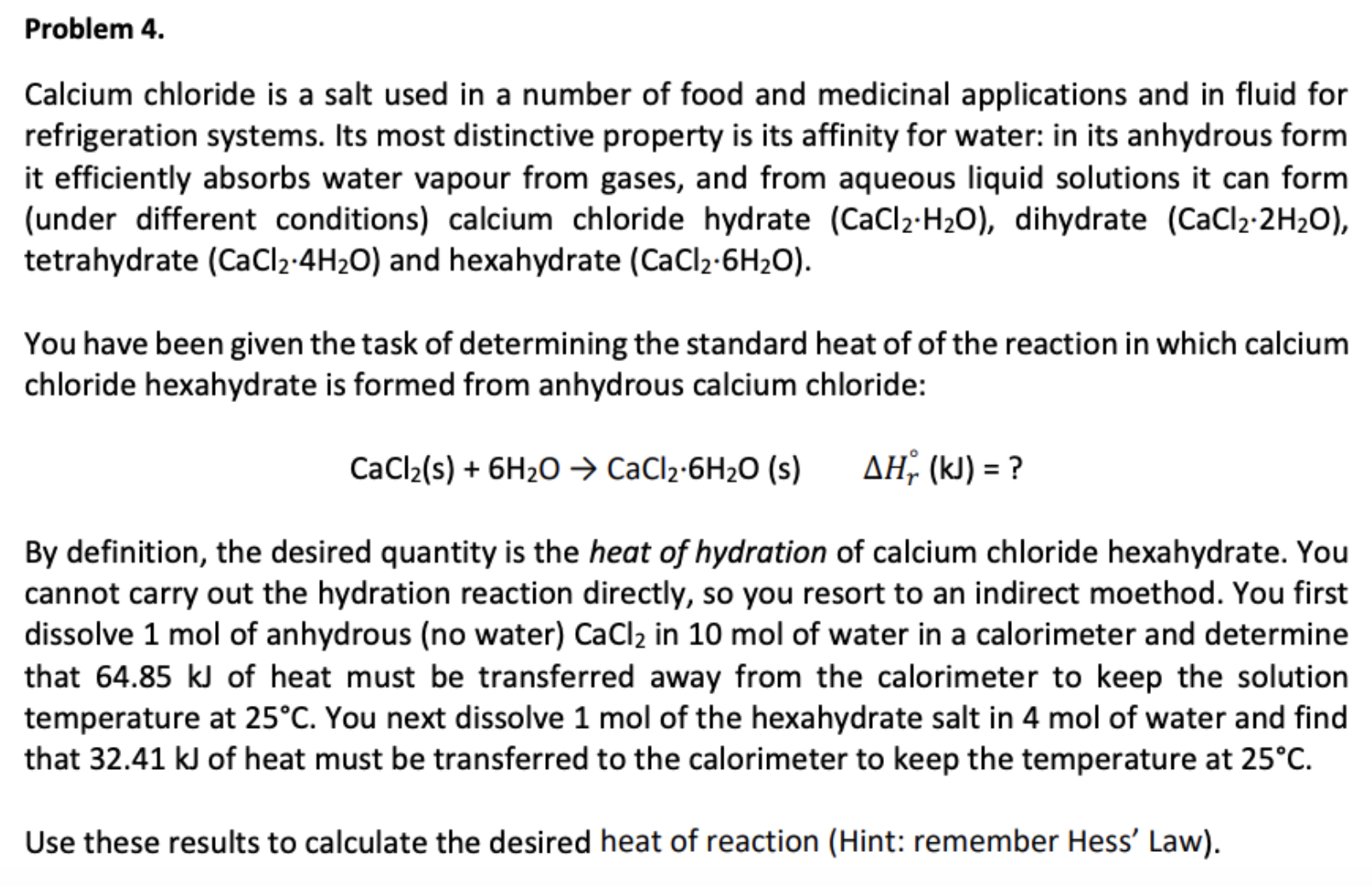
Transcribed Image Text:Problem 4.
Calcium chloride is a salt used in a number of food and medicinal applications and in fluid for
refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form
it efficiently absorbs water vapour from gases, and from aqueous liquid solutions it can form
(under different conditions) calcium chloride hydrate (CaCl2-H20), dihydrate (CaCl2 2H2O),
tetrahydrate (CaCl2-4H2O) and hexahydrate (CaCl2-6H2O).
You have been given the task of determining the standard heat of of the reaction in which calcium
chloride hexahydrate is formed from anhydrous calcium chloride:
AH (k) ?
CaCl2(s)6H20 CaCl2-6H20 (s)
By definition, the desired quantity is the heat of hydration of calcium chloride hexahydrate. You
cannot carry out the hydration reaction directly, so you resort to an indirect moethod. You first
dissolve 1 mol of anhydrous (no water) CaCl2 in 10 mol of water in a calorimeter and determine
that 64.85 kJ of heat must be transferred away from the calorimeter to keep the solution
temperature at 25°C. You next dissolve 1 mol of the hexahydrate salt in 4 mol of water and find
that 32.41 kJ of heat must be transferred to the calorimeter to keep the temperature at 25°C
Use these results to calculate the desired heat of reaction (Hint: remember Hess' Law)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
