Propose an efficient synthesis of the following compound using the malonic ester synthesis. Propose an efficient synthesis for the following transformation. DEt
Propose an efficient synthesis of the following compound using the malonic ester synthesis. Propose an efficient synthesis for the following transformation. DEt
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.25P: Show the product expected when the following unsaturated -ketoester is treated with each reagent....
Related questions
Question
100%
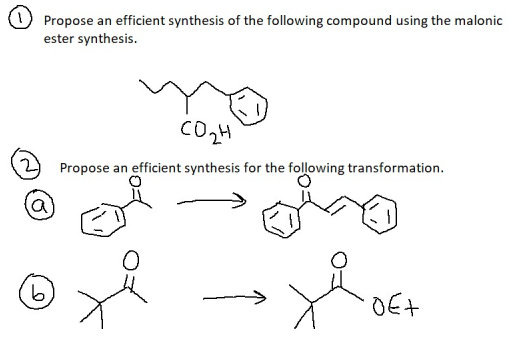
Transcribed Image Text:Propose an efficient synthesis of the following compound using the malonic
ester synthesis.
Propose an efficient synthesis for the following transformation.
DEt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning