Q: 1: The distribution constant for iodine between an organic solvent and H;O is 85. Find the concentration of I; remaining in the aqueous layer after extraction of 50.0ml. of 1.00 x 10 M I with the following quantities of the organic solvent: (a) 50.0 ml.; (b) two 25.0 ml. portions; (c) five 10.0 ml. portions.
Q: 1: The distribution constant for iodine between an organic solvent and H;O is 85. Find the concentration of I; remaining in the aqueous layer after extraction of 50.0ml. of 1.00 x 10 M I with the following quantities of the organic solvent: (a) 50.0 ml.; (b) two 25.0 ml. portions; (c) five 10.0 ml. portions.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
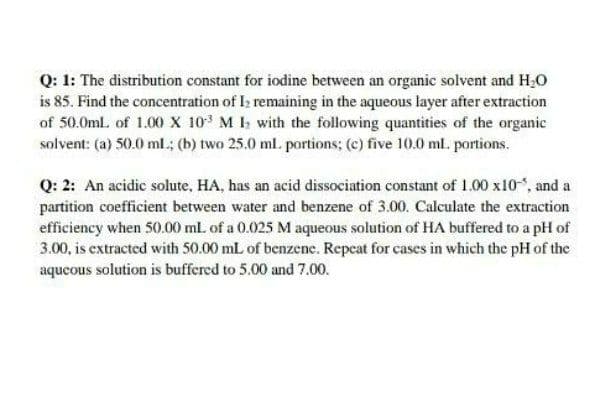
Transcribed Image Text:Q: 1: The distribution constant for iodine between an organic solvent and H,0
is 85. Find the concentration of I; remaining in the aqueous layer after extraction
of 50.0ml. of 1.00 x 10 M I with the following quantities of the organic
solvent: (a) 50.0 ml.; (b) two 25.0 ml. portions; (c) five 10.0 ml. portions.
Q: 2: An acidic solute, HA, has an acid dissociation constant of 1.00 x10, and a
partition coefficient between water and benzene of 3.00. Calculate the extraction
efficiency when 50.00 ml. of a 0.025 M aqueous solution of HA buffered to a pH of
3.00, is extracted with 50.00 mL of benzene. Repeat for cases in which the pH of the
aqueous solution is buffered to 5.00 and 7.00.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



