Q Search this cours References Use the References to access important values if needed for this question. According to the following reaction, how many grams of sodium iodide are required for the complete reaction of 29.6 grams of chlorine gas ? chlorine(g) + sodium iodide(s) sodium chloride(s) + iodine(s) grams sodium iodide Submit Answer chiometry of Rooctions: Gra. The is group attempt 1 of 5
Q Search this cours References Use the References to access important values if needed for this question. According to the following reaction, how many grams of sodium iodide are required for the complete reaction of 29.6 grams of chlorine gas ? chlorine(g) + sodium iodide(s) sodium chloride(s) + iodine(s) grams sodium iodide Submit Answer chiometry of Rooctions: Gra. The is group attempt 1 of 5
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 78AP: For each of the following balanced equations, indicate how many moles of the product could be...
Related questions
Question
Transcribed Image Text:Q Search this cours
References
Use the References to access important values if needed for this question.
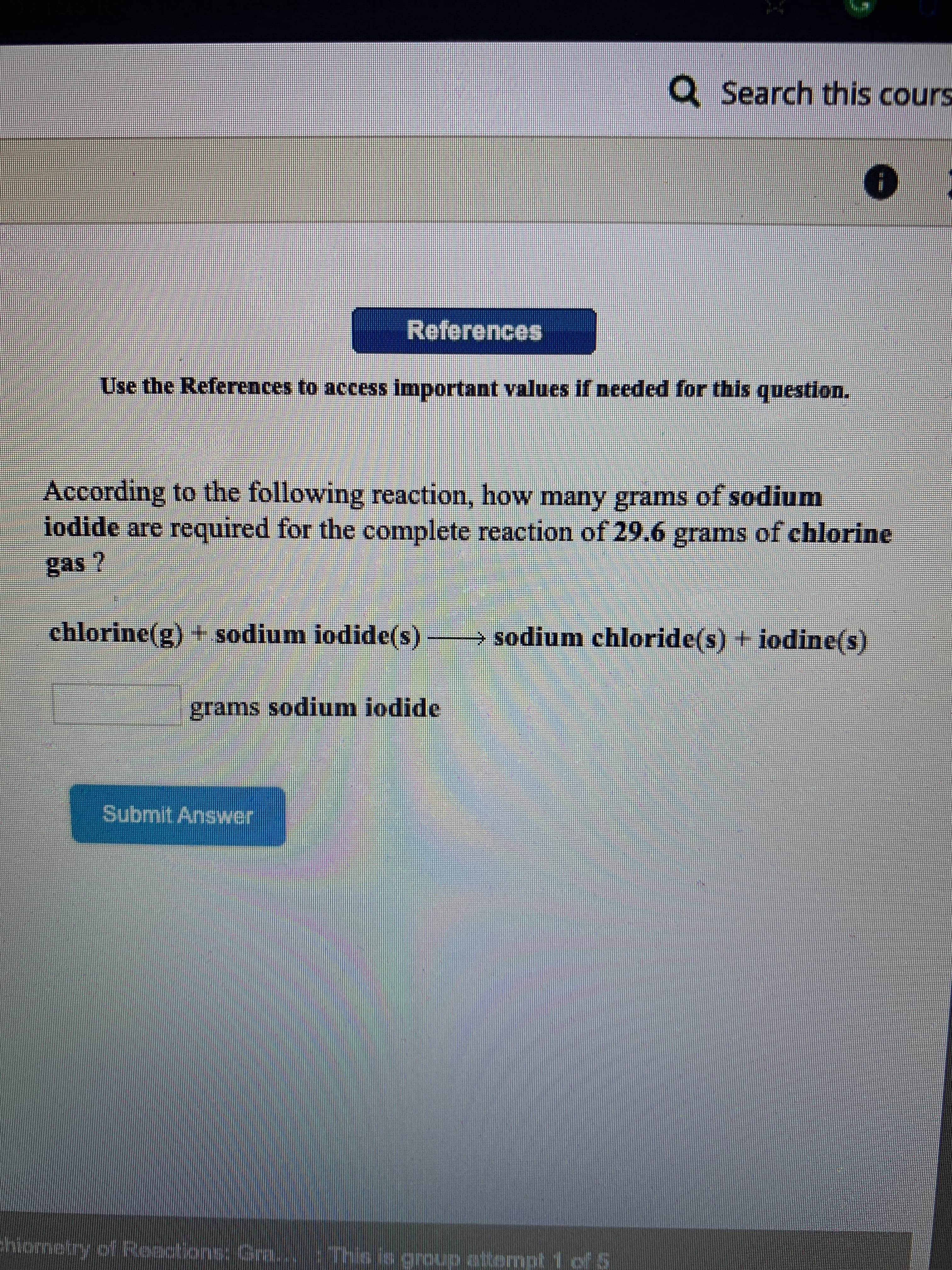
According to the following reaction, how many grams of sodium
iodide are required for the complete reaction of 29.6 grams of chlorine
gas ?
chlorine(g) + sodium iodide(s)
sodium chloride(s) + iodine(s)
grams sodium iodide
Submit Answer
chiometry of Rooctions: Gra.
The is group attempt 1 of 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning