Q1. Take the densities of water, oil, and mercury to be 1000 kg/m2,850 kg/m2, and 13,600 D = 2 m kg/m, respectively A cylindrical container, as shown in Figure 1, is filled by 1.1 m3 of granite, 1.15 m³ of rough sand and 84.69 fA³ of sea water at 25 C, 12.35 ft# is a carbon dioxide gas with density of 0.115 Ib/fte. The Paranite = 27500 mPsea water kg m•Psand = 1500 ke 1022 Find the (a) average specific volume and density when you include the air mass and volume. (b) specific volume based on mole for the carbon dioxide, the molecular weight is 44.01. 1.6 m .......
Q1. Take the densities of water, oil, and mercury to be 1000 kg/m2,850 kg/m2, and 13,600 D = 2 m kg/m, respectively A cylindrical container, as shown in Figure 1, is filled by 1.1 m3 of granite, 1.15 m³ of rough sand and 84.69 fA³ of sea water at 25 C, 12.35 ft# is a carbon dioxide gas with density of 0.115 Ib/fte. The Paranite = 27500 mPsea water kg m•Psand = 1500 ke 1022 Find the (a) average specific volume and density when you include the air mass and volume. (b) specific volume based on mole for the carbon dioxide, the molecular weight is 44.01. 1.6 m .......
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter1: Chemistry And Measurement
Section: Chapter Questions
Problem 1.37QP: A 15.5 g sample of sodium carbonate is added to a solution of acetic acid weighing 19.7 g. The two...
Related questions
Question
i need the answer quickly
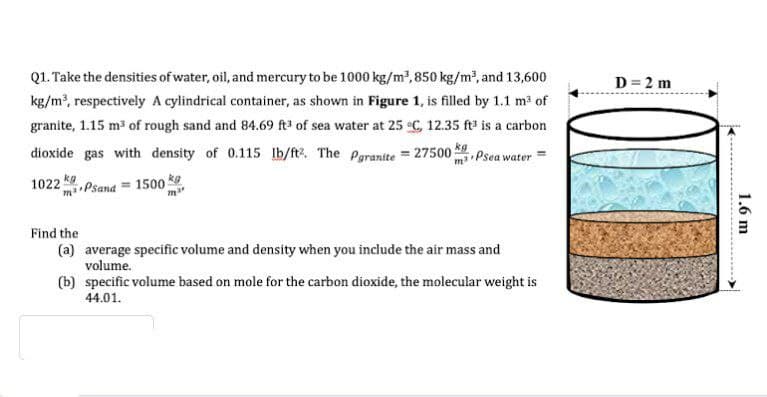
Transcribed Image Text:Q1. Take the densities of water, oil, and mercury to be 1000 kg/m²,850 kg/m2, and 13,600
D = 2 m
kg/m, respectively A cylindrical container, as shown in Figure 1, is filled by 1.1 m3 of
granite, 1.15 m3 of rough sand and 84.69 fA³ of sea water at 25 C, 12.35 ft# is a carbon
dioxide gas with density of 0.115 lb/fte. The Paranite = 27500
mi Psea water
kg
mi Psana = 1500 ke
1022
Find the
(a) average specific volume and density when you include the air mass and
volume.
(b) specific volume based on mole for the carbon dioxide, the molecular weight is
44.01.
1.6 m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning