Quantum mechanics predicts that the energy of the ground state of the H atom is –13.6 eV. Insight into the magni- tude of this quantity is gained by considering several methods by which it can be measured. (a) Calculate the longest wavelength of light that will ion- ize H atoms in their ground state. (b) Assume the atom is ionized by collision with an elec- tron that transfers all its kinetic energy to the atom in the ionization process. Calculate the speed of the electron before the collision. Express your answer in meters per second (m s-') and miles per hour (miles h-1). (c) Calculate the temperature required to ionize a H atom in its ground state by thermal excitation. (Hint: Recall the criterion for thermal excitation of an oscillator in Planck's theory of blackbody radiation is that hv z kµT.)
Quantum mechanics predicts that the energy of the ground state of the H atom is –13.6 eV. Insight into the magni- tude of this quantity is gained by considering several methods by which it can be measured. (a) Calculate the longest wavelength of light that will ion- ize H atoms in their ground state. (b) Assume the atom is ionized by collision with an elec- tron that transfers all its kinetic energy to the atom in the ionization process. Calculate the speed of the electron before the collision. Express your answer in meters per second (m s-') and miles per hour (miles h-1). (c) Calculate the temperature required to ionize a H atom in its ground state by thermal excitation. (Hint: Recall the criterion for thermal excitation of an oscillator in Planck's theory of blackbody radiation is that hv z kµT.)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 40P: When metallic sodium is dissolved in liquid sodium chloride, electrons are released into the liquid....
Related questions
Question
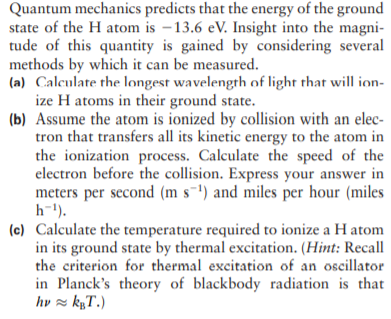
Transcribed Image Text:Quantum mechanics predicts that the energy of the ground
state of the H atom is –13.6 eV. Insight into the magni-
tude of this quantity is gained by considering several
methods by which it can be measured.
(a) Calculate the longest wavelength of light that will ion-
ize H atoms in their ground state.
(b) Assume the atom is ionized by collision with an elec-
tron that transfers all its kinetic energy to the atom in
the ionization process. Calculate the speed of the
electron before the collision. Express your answer in
meters per second (m s-') and miles per hour (miles
h-1).
(c) Calculate the temperature required to ionize a H atom
in its ground state by thermal excitation. (Hint: Recall
the criterion for thermal excitation of an oscillator
in Planck's theory of blackbody radiation is that
hv z kµT.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning