Question 14 of 29> < For the chemical reaction 3 KOH H, PO K,PO43 H2O how many moles of potassium phosphate will be produced from 43.9 g of potassium hydroxide? moles of potassium phosphate: mol help privacy policy terms of use about us contact us careers
Question 14 of 29> < For the chemical reaction 3 KOH H, PO K,PO43 H2O how many moles of potassium phosphate will be produced from 43.9 g of potassium hydroxide? moles of potassium phosphate: mol help privacy policy terms of use about us contact us careers
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 48QAP: Follow the instructions of Problem 47 for the following set of bulbs.
Related questions
Question
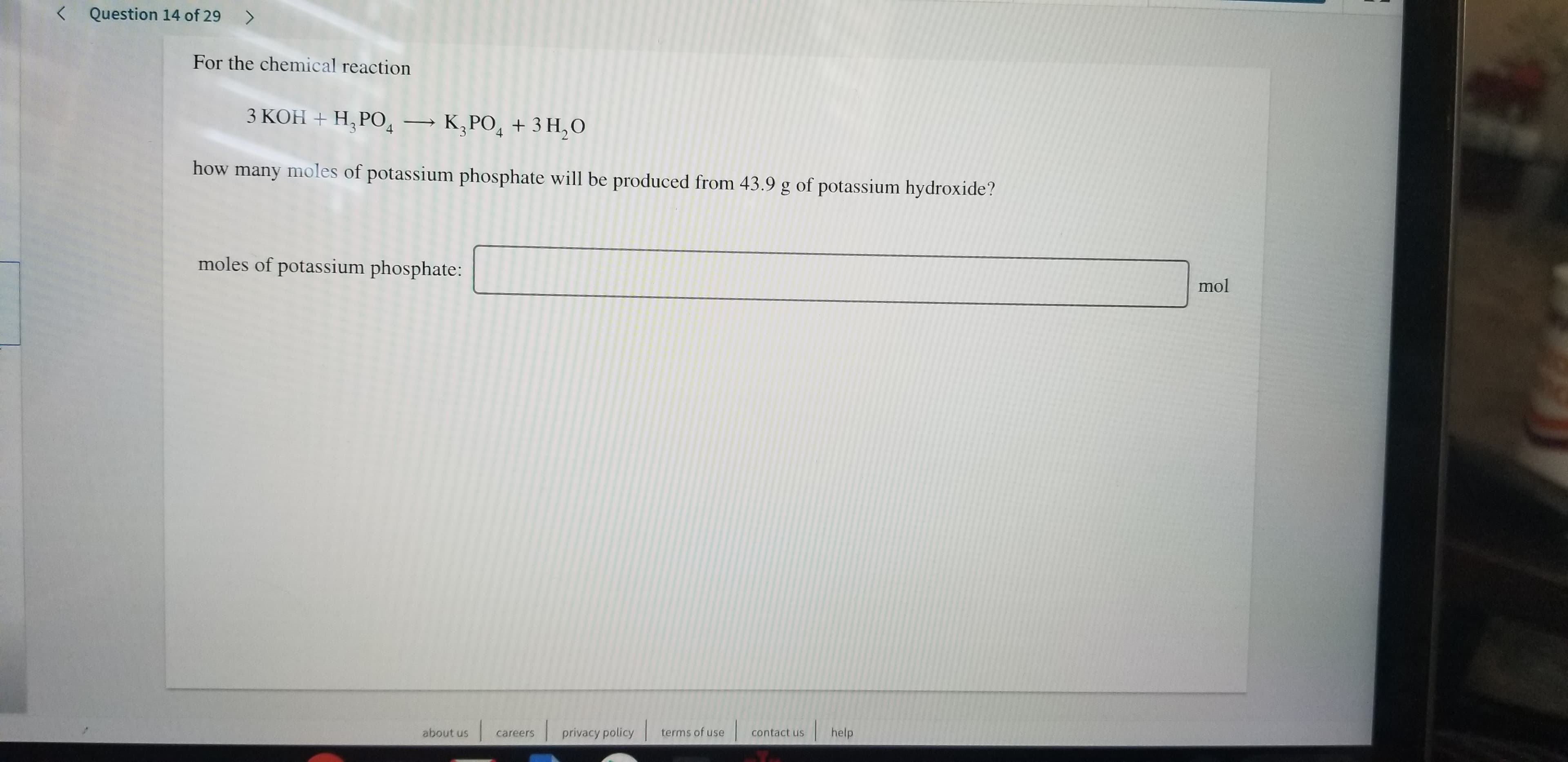
Transcribed Image Text:Question 14 of 29>
<
For the chemical reaction
3 KOH H, PO K,PO43 H2O
how many moles of potassium phosphate will be produced from 43.9 g of potassium hydroxide?
moles of potassium phosphate:
mol
help
privacy policy
terms of use
about us
contact us
careers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
