Question 2 A student has two samples of NaCl, each one from a different source. Assume that the only potential contaminant in eac' sample is KCl. The student runs an experiment to determine the percent by mass of chlorine in each sample. From the results of this experiment alone, which of the following questions is most likely to be answered? Which sample has the higher purity? B Which sample has the higher density? What is the source of the contaminants present in each of the samples? Which sample came from a salt mine, and which sample came from the ocean?
Question 2 A student has two samples of NaCl, each one from a different source. Assume that the only potential contaminant in eac' sample is KCl. The student runs an experiment to determine the percent by mass of chlorine in each sample. From the results of this experiment alone, which of the following questions is most likely to be answered? Which sample has the higher purity? B Which sample has the higher density? What is the source of the contaminants present in each of the samples? Which sample came from a salt mine, and which sample came from the ocean?
Glencoe Algebra 1, Student Edition, 9780079039897, 0079039898, 2018
18th Edition
ISBN:9780079039897
Author:Carter
Publisher:Carter
Chapter10: Statistics
Section10.1: Measures Of Center
Problem 9PPS
Related questions
Question
I want a concise explanation of why are those the correct answers
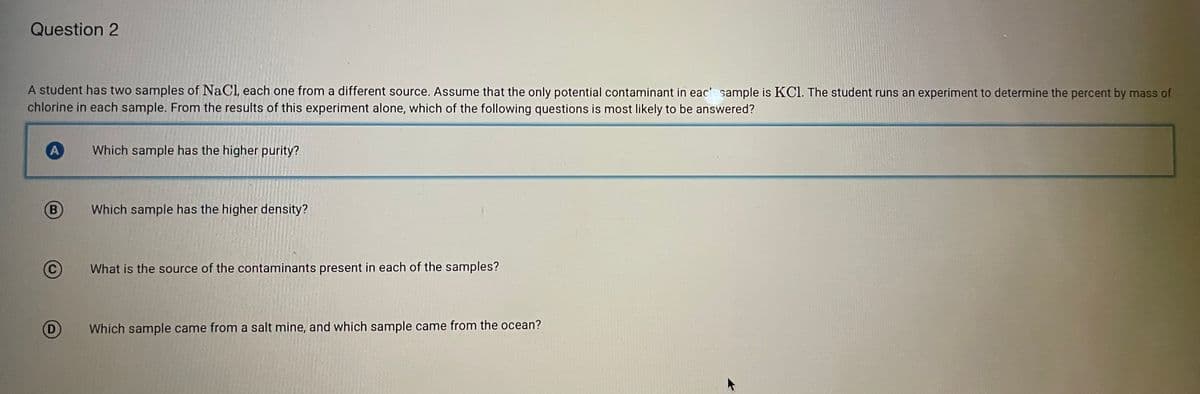
Transcribed Image Text:Question 2
A student has two samples of NaCl, each one from a different source. Assume that the only potential contaminant in eac sample is KCl. The student runs an experiment to determine the percent by mass of
chlorine in each sample. From the results of this experiment alone, which of the following questions is most likely to be answered?
Which sample has the higher purity?
B
Which sample has the higher density?
What is the source of the contaminants present in each of the samples?
(D)
Which sample came from a salt mine, and which sample came from the ocean?

Transcribed Image Text:Question 3
A student has samples of two pure compounds, XC1O3 and ZC103, which contain unknown alkali metals X and Z. The student measures the mass of each sample and then strongly heats the samples to
drive off all the oxygen, leaving solid residues of XCl and ZCl. The student measures the mass of the solid residue from each sample. Which of the following questions can be answered from the results of
the experiment?
Which has the greater molar mass, X or Z?
(B
Which has the higher boiling point, X or Z?
Which has the higher melting point, XCl or ZCl?
(D
Which has the greater density, XCl or ZCl?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning

Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning