Question 3: South Africa is one of the world's largest iron ore producers. From the reaction below, answer the following questions based on the iron ore extraction: Fe2O3(s) + C(s) Fe) + CO2(9) a) Give the correct chemistry name for Fe2O3 and CO2. b) If 9888.8g of Fe2O3 and 1258.2g of C react, determine which reagent is the limiting reagent/reactant of the reaction. c) Calculate theoretical yield of the reaction. d) If the foreman on site obtained 6.12 kg of Fe, for the reaction described in b) above, what is the % yield of the reaction? e) The product Fe is in liquid format. Do you think this is correct or not and justify why you think so?
Question 3: South Africa is one of the world's largest iron ore producers. From the reaction below, answer the following questions based on the iron ore extraction: Fe2O3(s) + C(s) Fe) + CO2(9) a) Give the correct chemistry name for Fe2O3 and CO2. b) If 9888.8g of Fe2O3 and 1258.2g of C react, determine which reagent is the limiting reagent/reactant of the reaction. c) Calculate theoretical yield of the reaction. d) If the foreman on site obtained 6.12 kg of Fe, for the reaction described in b) above, what is the % yield of the reaction? e) The product Fe is in liquid format. Do you think this is correct or not and justify why you think so?
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter6: Properties Of Hydrates
Section: Chapter Questions
Problem 1ASA: A student is given a sample of a pink manganese (II) chloride hydrate. She weighs the sample in a...
Related questions
Question
Q3
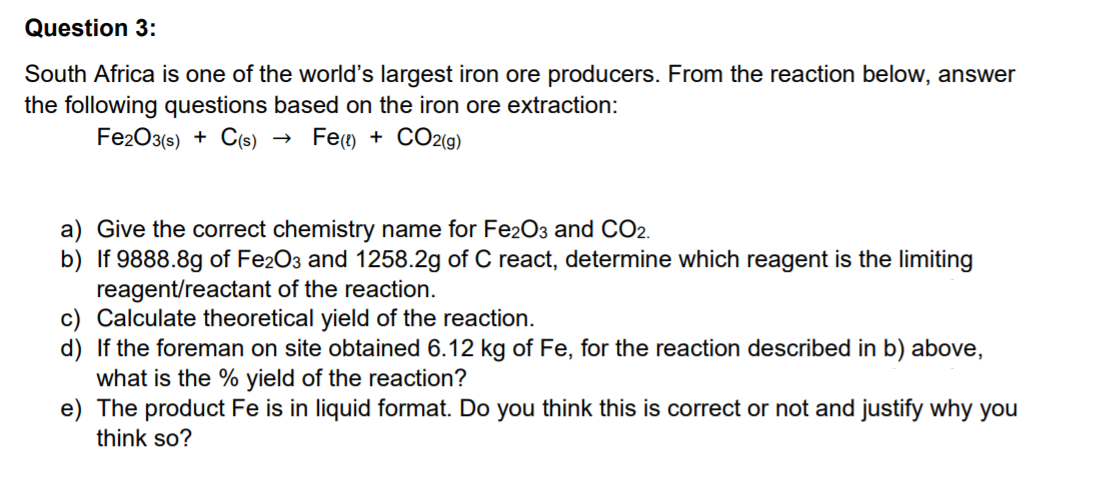
Transcribed Image Text:Question 3:
South Africa is one of the world's largest iron ore producers. From the reaction below, answer
the following questions based on the iron ore extraction:
Fe2O3(s) + C(s)
Fe() + CO2(g)
a) Give the correct chemistry name for Fe2O3 and CO2.
b) If 9888.8g of Fe2O3 and 1258.2g of C react, determine which reagent is the limiting
reagent/reactant of the reaction.
c) Calculate theoretical yield of the reaction.
d) If the foreman on site obtained 6.12 kg of Fe, for the reaction described in b) above,
what is the % yield of the reaction?
e) The product Fe is in liquid format. Do you think this is correct or not and justify why you
think so?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning