QUESTION5 Which Fischer projection has an absolute configuration of R? B. C. D. CH2CH3 CH2CH3 CH CH2 CH3CH2-1-Br CH=CH2 CH2CH3 Br O A. structure A O B. structure B C. structure C D. structure D
QUESTION5 Which Fischer projection has an absolute configuration of R? B. C. D. CH2CH3 CH2CH3 CH CH2 CH3CH2-1-Br CH=CH2 CH2CH3 Br O A. structure A O B. structure B C. structure C D. structure D
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 37CTQ: Indicate the relationship between each pair. Choose from: configurational stereoisomers,conformers,...
Related questions
Question
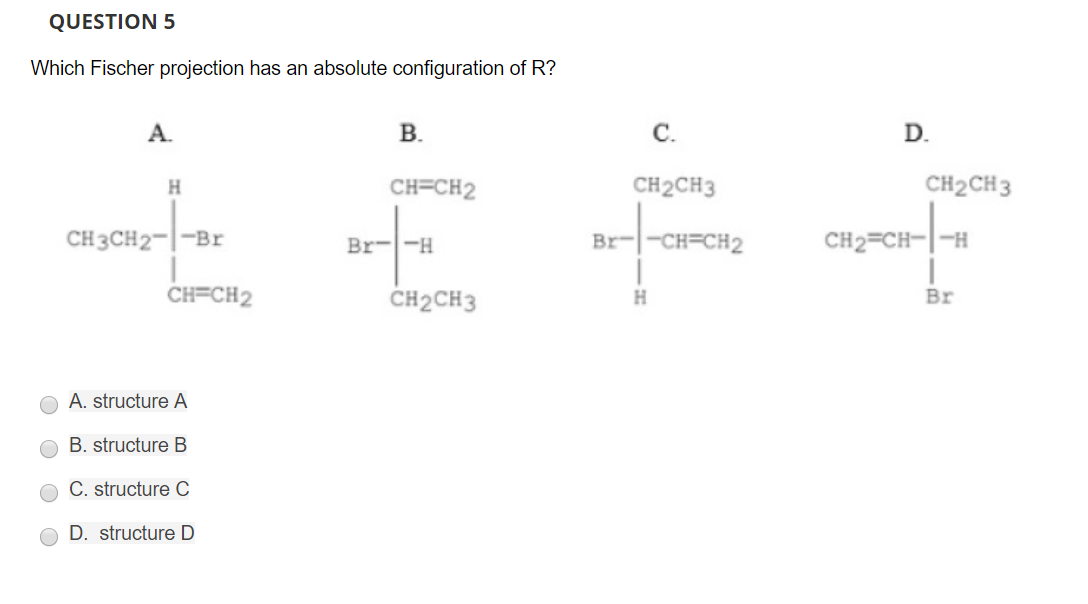
Transcribed Image Text:QUESTION5
Which Fischer projection has an absolute configuration of R?
B.
C.
D.
CH2CH3
CH2CH3
CH CH2
CH3CH2-1-Br
CH=CH2
CH2CH3
Br
O A. structure A
O B. structure B
C. structure C
D. structure D
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 4 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning
