R 2 3 4 T 5 CM 1 10 11 12 13 14 PERIOD ACTINOIDS LANTHANOIDS 89 Chromatography 66 22 ns 11 12 Na Mg (No date wthout mpioe: Magnesium 662 24.31 61P Ca Sc 21 22 24 Cr Potassium Calclum Scandium Titanium Vanadium Chremium 44.96 47.87 50.94 52.00 eidi ch 39.10 40.08 Name 38 41 42 Zr Nb Mo 7 OV 30-9:20 Rb Sr Rubidium Strontium Zirconium Niobium 95.95 wnuepq 85.47 87.62 88.91 91.22 92.91 hit: 55 72 73 74 Cs Ba 965 Hf Та 57-71 Cesium Barium Hafnium Tantalum Tungsten 132.91 137.33 178.49 180.95 183.84 90 Rf Db Sg 87 88 104 105 1. A representation of a developed paper chr Fr Ra 89-103 Francium Radium Rutherfordium Dubnium Seaborgi ALKALI METALS 4:2 57 58 09 La Ce Се Pr Nd Praseodymium Neodymium 144.24 Lanthanum 138.91 140.12 140.91 91 92 06 Ac Th Pa Actinium Thorium Protactinium Uranium 9.1 232.04 231.04 238.0 Notes: "Caesium" and "aluminium are the i atomic mass) of an element from a specifie atom of 12C (IUPAC). Sources: lUPAC peric 19 A BC Use a ruler to determine the R, values for 0.5 BU.5 L010 0.S HOM 7'h
R 2 3 4 T 5 CM 1 10 11 12 13 14 PERIOD ACTINOIDS LANTHANOIDS 89 Chromatography 66 22 ns 11 12 Na Mg (No date wthout mpioe: Magnesium 662 24.31 61P Ca Sc 21 22 24 Cr Potassium Calclum Scandium Titanium Vanadium Chremium 44.96 47.87 50.94 52.00 eidi ch 39.10 40.08 Name 38 41 42 Zr Nb Mo 7 OV 30-9:20 Rb Sr Rubidium Strontium Zirconium Niobium 95.95 wnuepq 85.47 87.62 88.91 91.22 92.91 hit: 55 72 73 74 Cs Ba 965 Hf Та 57-71 Cesium Barium Hafnium Tantalum Tungsten 132.91 137.33 178.49 180.95 183.84 90 Rf Db Sg 87 88 104 105 1. A representation of a developed paper chr Fr Ra 89-103 Francium Radium Rutherfordium Dubnium Seaborgi ALKALI METALS 4:2 57 58 09 La Ce Се Pr Nd Praseodymium Neodymium 144.24 Lanthanum 138.91 140.12 140.91 91 92 06 Ac Th Pa Actinium Thorium Protactinium Uranium 9.1 232.04 231.04 238.0 Notes: "Caesium" and "aluminium are the i atomic mass) of an element from a specifie atom of 12C (IUPAC). Sources: lUPAC peric 19 A BC Use a ruler to determine the R, values for 0.5 BU.5 L010 0.S HOM 7'h
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter20: Carbohydrates
Section: Chapter Questions
Problem 20.56P
Related questions
Question
It ask that I use a rulet to determine the Rf values for each dye in the mixture. Is this correct?
Also explain why all the Rf values should be between 0 and 1?
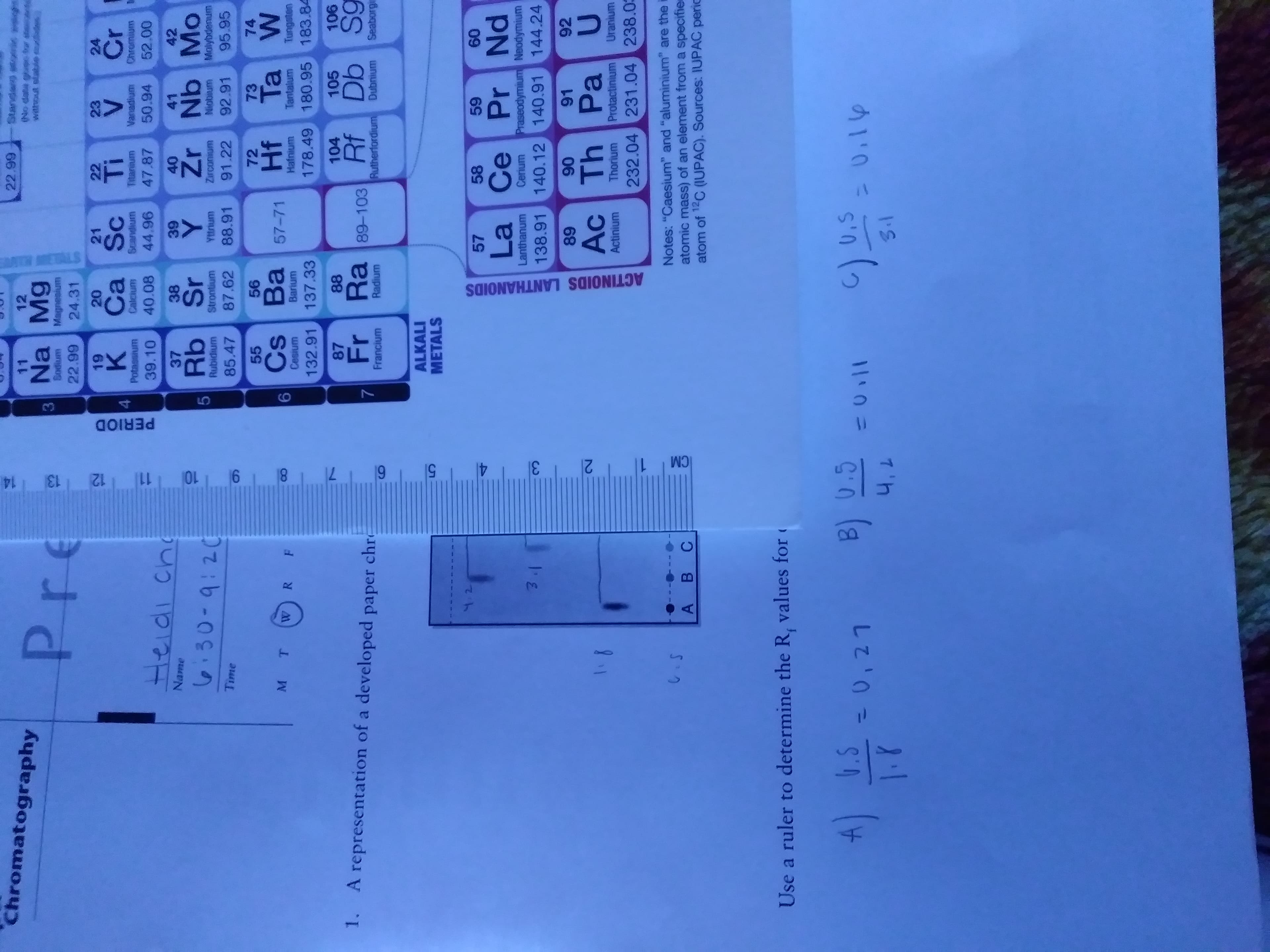
Transcribed Image Text:R
2 3 4 T 5
CM 1
10 11
12
13 14
PERIOD
ACTINOIDS LANTHANOIDS
89
Chromatography
66 22
ns
11
12
Na Mg
(No date
wthout
mpioe:
Magnesium
662
24.31
61P
Ca Sc
21
22
24
Cr
Potassium
Calclum
Scandium
Titanium
Vanadium
Chremium
44.96
47.87
50.94
52.00
eidi ch
39.10
40.08
Name
38
41
42
Zr Nb Mo
7
OV
30-9:20
Rb Sr
Rubidium
Strontium
Zirconium
Niobium
95.95
wnuepq
85.47
87.62
88.91
91.22
92.91
hit:
55
72
73
74
Cs Ba
965
Hf
Та
57-71
Cesium
Barium
Hafnium
Tantalum
Tungsten
132.91 137.33
178.49 180.95 183.84
90
Rf Db Sg
87
88
104
105
1. A representation of a developed paper chr
Fr
Ra
89-103
Francium
Radium
Rutherfordium
Dubnium
Seaborgi
ALKALI
METALS
4:2
57
58
09
La Ce
Се
Pr Nd
Praseodymium Neodymium
144.24
Lanthanum
138.91
140.12 140.91
91
92
06
Ac Th Pa
Actinium
Thorium
Protactinium
Uranium
9.1
232.04 231.04 238.0
Notes: "Caesium" and "aluminium are the i
atomic mass) of an element from a specifie
atom of 12C (IUPAC). Sources: lUPAC peric
19
A BC
Use a ruler to determine the R, values for
0.5
BU.5
L010
0.S
HOM
7'h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning