reactant: 0.15g of p-amionphenol (MW=109.1) 0.165 ml of acetic anhyride (mw= 102.1 d= 1.08 ml) product: acetaminophen mw = 151.2 Calculate the E-factor of the process (70% actual yield).
Q: The additive in gasoline was measured six times with the following results: 0.13, 0.12, 0.16, 0.17,…
A:
Q: The literature value for the Ksp of Ca(OH)2 is 5.5e-6 at 25 oC. How does this compare with the value…
A: The Ksp value of calcium hydroxide is 5.5×10-6 and given that value obtained from the experiment is…
Q: dm3 Reaction rate specification ,K ( mol. min FA0 – FAI = -rAV kCA? = -TA voCAO – v0 CAI = kCA1?V vo…
A: We have the second-order reaction 2A→Product Rate expression for this reaction is -rA=KCA2 Mass…
Q: Organic carbon in seawater can be measured by oxidation to CO2 with K2S2O8, followed by gravimetric…
A:
Q: A fermenter was filled with 10L of 0.6 mol/L sodium sulfite solution containing 0.003M Cu2+ ion and…
A: Sodium sulfite is oxidized to sodium sulfate in the presence of a catalyst, Cu2+ or Co2+ which is…
Q: Quanktahre Amalyais : Ex: A constant eectic cunent depasite 365 mg. 9 Ag in 216 mimutes yom an…
A: Given that: amount deposited= 365 mg t = 216 min To find: the amount of current?
Q: A pilot plant reactor was charged with 50 kg naphthalene and 200 kg (98% by wt) H2SO4. The reaction…
A: Complete analysis of the products can be obtained by calculating the mass formed of each product.
Q: 10.5mL (mw 106) of benzaldehyde and 2.9g(3.63mL, mw 40) of acetone react with 5g of NaOH and 25mL of…
A:
Q: A 0.1475-M solution of Ba(OH)2 was used to titrate the acetic acid (60.05 g/mol) in a dilute aqueous…
A: w/v percentage of acetic acid in each of the sample 1 to 4 are required to calculate the mean w/v…
Q: A chemical factory has been illegally disposing their chemical wastes without necessary…
A:
Q: 4) How would your calculated value of Kup be affected by errors introduced from the following…
A: Solubility product constant (equilibrium constant, Ksp) designates the attainment of equilibrium…
Q: Calculate the yield of tert- butyl chloride if we take 7ml of tert- butanol and the actual mass of…
A: Given in questiin: Moles ratio =1:1 Volume of tert-butanol = 7mL Molecular weight of tert-butanol…
Q: Initial temp: 19.06 C trial 1: 0.5 M concentration trial 2 : 1.0 M concentration 200 mL of HCl…
A: trial 1: 0.5 M concentration 200 mL of HCl and 200 mL of NaOH are combined in an insulated…
Q: Calculate the 90% confidence limit for the mean of the data assuming that only information about the…
A:
Q: Potential energy at top Ep = mgh E = 300 kg Ep=147000 J 9.8 m/s Reminder 100 km 27.8 m/s CALCULATE
A: Given -> Mass (m)= 300 kg Acceleration due to gravity (g) = 9.8 m/s2 Height (h) = 50 m
Q: 7. What effect will a 1.0°C error (all temperature readings are 1.0°C greater than the actual value)…
A: If regarding 1°C greater than the actual value for the final temperature ΔT will be greater than the…
Q: Which of the following are equivalent to 2,500 ppm Cu2+? (There may be more than one answer)…
A: Given data 2500 ppm equal to
Q: The temperature of a reaction was monitored with an thermometer that is reported to have precision…
A: Expanded uncertainty can be calculated as follows: U=kuc(y) where k: Coverage factor (2) uc(y):…
Q: नि 360 8 वैकगोe ्न N-2 - ৭cलकmाdo- 2-ammochane . ि वतंवे ए० उचडइापना ठेाक (मCहS+ ल वीरळना/ंगन्न क…
A:
Q: Full Screen Accessibility The Ksp of the phosphate fertilizer CaHPO4 2H20 is 2.7 x 10-7 at 25°C. The…
A: Given: Ksp=2.7 x 10-7 CaHPO4.2H2O --> Ca2+ + HPO42- + 2 H2O s…
Q: 7A + 413 A. rate equation PA Rate = K[A] [B] [4] P B. Calculate [A] 1.7 2. 1.4 3..7 4. .7 [B] 1.3…
A: Here we are required to find the rate constant for the given reaction.
Q: Need the theoretical yield for following Starring: 1,4 Pimethoxy Bentene : 0.321 grams Mw=138.14…
A:
Q: 13.43 Octane (C3H18) enters an engine and burns with air to give products with the dry molar…
A:
Q: What is the std heat of reaction at 800 degrees celsius (1073.15K) for a complete combustion of…
A: We cannot calculate directly without any data. We know standard heat of reaction at standard…
Q: What is ΔSsurr for a reaction at 28.6 °C with ΔHsys = 38.9 kJ mol-1 ? Express your answer in J…
A: We have : ΔHsys = 38.9 kJ mol-1= 38900 J mol-1 => ΔHsurr = - 38900 J mol-1 T= 28.6°C = (28.6 +…
Q: In an experiment with an isolated segment of the ascending loop of Henle, methoxyinulin was added to…
A: Calculation of Vout: Cin×Vin=Cout×Vout1000 cpm×2 nL/min=1400 cpm×VoutVout=1.4286 nL/min
Q: Some wastewater stream contains 0.015 M Hg22+. Some chemical engineer decides to remove it by…
A: Concentration of Hg22+ in waste water = 0.015 M Volume of waste water taken = 100 mL Concentration…
Q: -.42. The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a…
A: Solved in step 2 and 3.
Q: You are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role…
A: In the case of uncompetitive inhibition, Ki for the inhibitor is calculated as shown in equation…
Q: What is the magnitude of teh uncertainity associated with each of the following measurements. a)…
A: The quantities which come across during the scientific studies are named as physical quantities. The…
Q: 1. In the following reaction, 325 mg of 2-hydroxybenzoic acid are reacted with 150 ul of methanol.…
A:
Q: < E session.masteringchemistry.com MasteringChemistry: HW 8 <HW 8 Applications for Dilution…
A: From the given data in the question, sayThe final concentration of the diluted solution = cVolume…
Q: Lactated Ringer’s/5% Dextrose solution contains: 6 g/L of Sodium Chloride (NaCl MW 58.5) 3.1 g/L of…
A: Each KCl forma 1 potassium ion, K+. Hence mEq/mL of potassium ion is the same as mEq/mL of KCl…
Q: A wet pipette was used to transfer 10.00 mL of 1M HNO3 in the determination of ΔHrxn. [magnitude of…
A: Since you have posted multiple questions, we are entitled to answer the first only.
Q: calculate the value of the equilibriomconstant, Rc₂ Q (g) + x (g) = 2 M(g) + N(0) given that M Meg)…
A:
Q: Can you work our KSP for me? thanks Liquid Amount - How many grams of each of the following…
A: Here given that the The solubility is 0.123 g/100 mL So substance dissolve in 4.70×102 mL of cold…
Q: A student performed the experiment described in this module, using 5.00 mL of a 3.2% H2O2 solution…
A: To calculate the percentage error, we need to find the gas constant (R) value obtained from the…
Q: A certain drug has a half-Iife in the body of 2.5 h. What should the interval between doses be, if…
A: The half-life of the drug in the body is = 2.5 h The interval between doses be, if the concentration…
Q: Consider the calcination of CaCO;: CaCO3 -> CaQ + CO2 Before calcination, the initial weight of…
A: The reaction is process by the reaction between two or more reactant to each other. The mass of each…
Q: 1-arr In a study of the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30…
A:
Q: 1.A student performed an experiment to find the number of water molecules associated w CuSO, x H,O,…
A: For CuSO4. xH2O. Mass of hydrated CuSO4= (mass of micro crucible +hydrate) - (mass of empty micro…
Q: Organic carbon in seawater can be measured by oxidation to CO2 with K2S2O8, followed by gravimetric…
A: Molar mass is defined as an average mass of atoms present in the chemical formula. It is the sum of…
Q: A research group discovers a new version of happyase, which they call happyase*, that catalyzes the…
A: According to the question - the chemical reaction is: HAPPY ⇌ SAD.
Q: Data Table 1: Gas volumes observed at 15 minutes Interval Sugar Initial 15 30 45 60 Total Glucose…
A: In presence of oxygen gas, glucose is converted into CO2 and water. In this process, 38 ATPs are…
Q: A student performed the experiment described in this module, using 7.00 mL of a 1.8% H2O2 solution…
A: First calculating the H2O2: 1.8% H2O2 solution means 1.8 g H2O2 in 100 ml of watermass in 7 ml = 1.8…
Q: A soil is analyzed for total iron content by dissolving 0.1 g of soil in 10 mL of acid and digesting…
A: Amount of soil = 0.1 g Final volume of solution to be analyzed = 100 ml Mass of iron present in 1000…
Q: What is the literature value of the Ksp of Ca(OH)2 ? How far off from this value was your value(s)…
A: Ksp is defined as the solubility product and the general expression for solubility product is shown…
Q: What is the capital cost of a plant that produces 250,000 tons per year of ethanol (fuel grade)…
A: Ans) $ 149.89 M
Q: Part I. Cause and Effect: ldentify the effect of the condition on the indicated parameter. Write…
A: Enthalpy of reaction is the change in heat during a chemical reaction and can be determined by…
Q: eactant: 0.400g of p-amionphenol (MW=109.1) 0.450 ml of acetic anhyride (mw= 102.1 d= 1.08 ml)…
A: Given : actual yield = 78% mass of acetaminophene = 151.2 g mass of P-aminophenol = 0.400 g (MW =…
reactant:
0.15g of p-amionphenol (MW=109.1)
0.165 ml of acetic anhyride (mw= 102.1 d= 1.08 ml)
product:
acetaminophen mw = 151.2
- Calculate the E-factor of the process (70% actual yield).
The chemical equation for the reaction between the given reactants is;
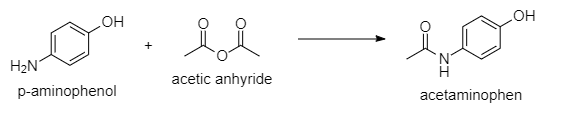
The number of moles of p-aminophenol and acetic anhydride are;
Step by step
Solved in 4 steps with 1 images

- You are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role in liver disease. In the laboratory the enzyme was shown to have a Km of 1.0 x 10-6 M and Vmax of 0.1 micromoles/min.mg measured at room temperature. You developed a competitive inhibitor. In the presence of 5.0 x 10-5 M inhibitor, the apparent Km of the enzyme was found to be 1.5 x 10-5 M. What is the Ki of the inhibitor?You are trying to come up with a drug to inhibit the activity of an enzyme thought to have a role in liver disease. In the laboratory the enzyme was shown to have a Km of 1.0 x 10-6 M and Vmax of 0.1 micromoles/min.mg measured at room temperature. You developed an uncompetitive inhibitor. In the presence of 5.0 x 10-5 M inhibitor, the apparent Vmax was determined to be 0.02 micromoles/min.mg. What is the Ki of the inhibitor?Chemistry The levels of an organic pollutant (P) in the groundwater at the perimeter of a plant were a cause for concern. A 10 mL sample of the water was taken and the pollutant was extracted with 95% efficiency using 25 mL of diethyl ether. GC was used to analyse the concentration of P in diethyl ether. A calibration curve was plotted for a series of standards of P which yielded the following results: Peak Area Toluene Conc. (µg/ml) 12,000 2.6 23,700 5.0 35,500 7.7 46,800 9.9 31,250 Sample Determine the concentration of P in ppb in the initial groundwater sample.
- What is ΔSsurr for a reaction at 28.6 °C with ΔHsys = 38.9 kJ mol-1 ? Express your answer in J mol-1 K-1 to at least two significant figures.Spike recovery and detection limit. Species of arsenic found in drinking water include AsO32 3 (arsenite), AsO32 4 (arsenate), (CH3)2AsO2 2 (dimethylarsinate), and (CH3)AsO22 3 (methylarsonate). Pure water containing no arsenic was spiked with 0.40 mg arsenate/L. Seven replicate determinations gave 0.39, 0.40, 0.38, 0.41, 0.36, 0.35, and 0.39 mg/L.15 Find the mean percent recovery of the spike and the concentration detection limit (mg/L).10.5mL (mw 106) of benzaldehyde and 2.9g(3.63mL, mw 40) of acetone react with 5g of NaOH and 25mL of Ethanol to form 6.7g of Dibenzalacetone. What is the theoretical, actual and percent yield?
- Lactated Ringer’s/5% Dextrose solution contains: 6 g/L of Sodium Chloride (NaCl MW 58.5) 3.1 g/L of Sodium Lactate (C3H5O3Na MW 112) 0.3g/L of Potassium Chloride (KCl MW 74.5) 0.2g/L of Calcium Chloride (CaCl2•2H2O MW 147) 50g/L of Dextrose (C6H12O6 MW 180) You receive an order to increase the Potassium ion concentration to 0.045 mEq/mL. How many mL of 14.9% Potassium chloride injection should be added to 1L of the above solution to increase the potassium ion concentration to 0.045 mEq/mL ____________________mL 14.9% KCl injectionWhat is the theoretical yield of ethyl chloride in the reaction of 20.2 gg of ethylene with 48 gg of hydrogen chloride? (For ethylene, MW=28.0amuMW=28.0amu; for hydrogen chloride, MW=36.5amuMW=36.5amu; for ethyl chloride, MW=64.5amuMW=64.5amu.)H2C=CH2+HCl→CH3CH2ClH2C=CH2+HCl→CH3CH2ClOrganic carbon in seawater can be measured by oxidation to CO2 with K2S2O8, followed by gravimetric determina- tion of CO2 trapped by a column of Ascarite. Water weighing 6.234 g produced 2.378 mg of CO2 (FM 44.010). Find ppm carbon in the seawater.
- If exactly 200.0 mL of an aqueous solution that is 1.037E3 ppb Pb(NO3)2 (331.2 g/mol) is mixed with 100.0 mL of an aqueous solution that is 3.090E9 fM KI (166.00 g/mol), how many micrograms of solid PbI2 (461.01 g/mol, 6.16 g/mL) are formed? Assume complete reaction.A 0.1475-M solution of Ba(OH)2 was used to titrate the acetic acid (60.05 g/mol) in a dilute aqueous solution. The following results were obtained. (See attached image)(a) Calculate the mean w/v percentage of acetic acid in the sample.(b) Calculate the standard deviation for the results.(c) Calculate the 90% confidence interval for the mean.(d) (d) At the 90% confidence level, could any of the results be discarded?Qq.1. Subject :- Account

