[References [Review Topicbs) Classify the following compounds as chiral, achiral (but not meso), or meso. 1st structure: OH H-HOH CO2H 2nd structure: CO2H Visited Br 3rd structure: H CI Retry Entire Group Submit Answer 6 more group atternpts remaining e I ---HI
[References [Review Topicbs) Classify the following compounds as chiral, achiral (but not meso), or meso. 1st structure: OH H-HOH CO2H 2nd structure: CO2H Visited Br 3rd structure: H CI Retry Entire Group Submit Answer 6 more group atternpts remaining e I ---HI
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 6E
Related questions
Question
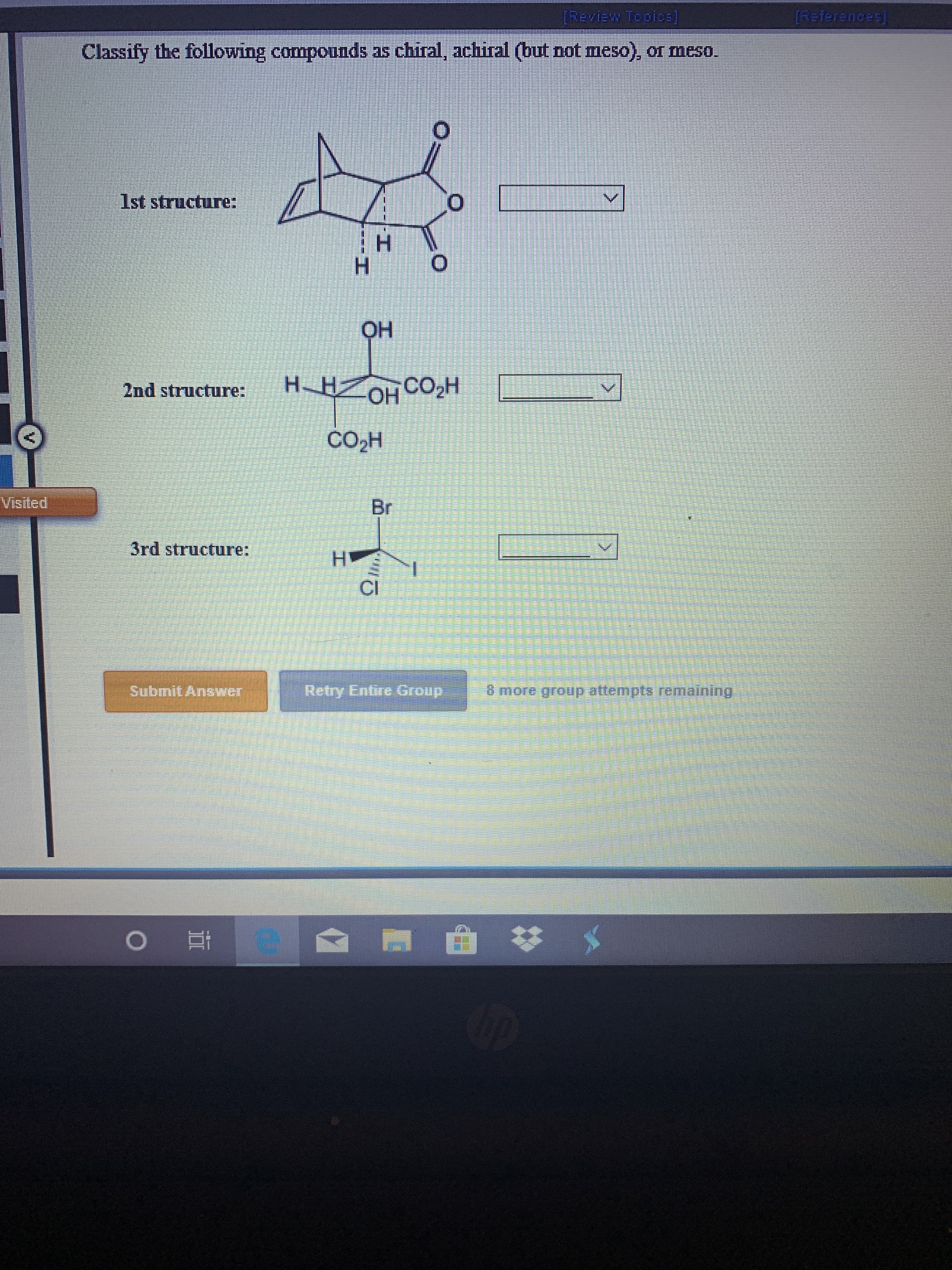
Transcribed Image Text:[References
[Review Topicbs)
Classify the following compounds as chiral, achiral (but not meso), or meso.
1st structure:
OH
H-HOH CO2H
2nd structure:
CO2H
Visited
Br
3rd structure:
H
CI
Retry Entire Group
Submit Answer
6 more group atternpts remaining
e
I
---HI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning