Rep Click the "draw structure" button to launch the drawing utility. Draw the skeletal structure of the principal organic product for the reaction below. CH3 O SOCI2 pyridine draw structure OH Next> 4 of. 11
Rep Click the "draw structure" button to launch the drawing utility. Draw the skeletal structure of the principal organic product for the reaction below. CH3 O SOCI2 pyridine draw structure OH Next> 4 of. 11
Chapter98: Guide To The Chemical Literature
Section: Chapter Questions
Problem 5P
Related questions
Question
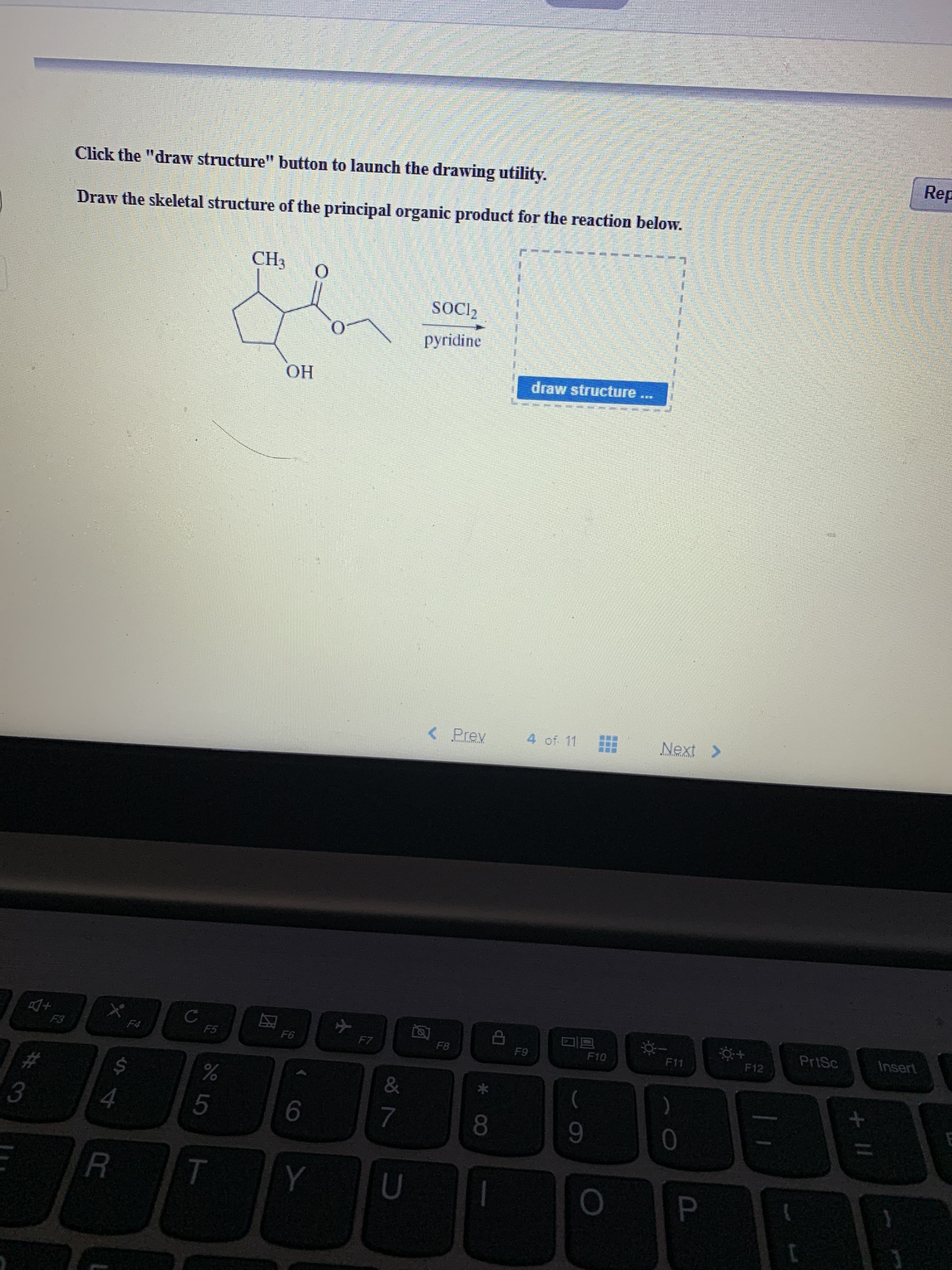
Transcribed Image Text:Rep
Click the "draw structure" button to launch the drawing utility.
Draw the skeletal structure of the principal organic product for the reaction below.
CH3
O
SOCI2
pyridine
draw structure
OH
Next>
4 of. 11
<Prev
Insert
PrtSc
X
F12
F11
F10
F9
F8
F7
F6
F5
F4
F3
)
&
#
0
7
4
3
P
U
Y
T
R
+II
LO
ST
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning