Review I Constants I Periodic Table Calorimetry is a method used to measure changes in enthalpy, or heat, that occur during chemical processes. Two common calorimeters are constant-pressure calorimeters and constant- volume (or "bomb") calorimeters. Bomb calorimeters are used to measure combustion and other gas-producing reactions, in which the reaction is observed in a strong, sealed vessel. A simple Part A In the following experiment, a coffee-cup calorimeter containing 100 mL of H20 is used. The initial temperature of the calorimeter is 23.0 °C. If 9.100 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution AHsoln of CaCl2 is -82.8 kJ/mol. constant-pressure calorimeter can be made from a foam coffee cup and a thermometer, in which energy changes in a reaction are observed via the change in temperature of the solution in the cup. The idea behind calorimeters is that if they are sufficiently insulated from the outside environment, any energy gained or lost in the chemical reaction will be directly observable as a temperature and/or pressure change in the calorimeter. Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs = 4.184 J/g .° C. Express your answer with the appropriate units. • View Available Hint(s) HẢ Value Units Submit
Review I Constants I Periodic Table Calorimetry is a method used to measure changes in enthalpy, or heat, that occur during chemical processes. Two common calorimeters are constant-pressure calorimeters and constant- volume (or "bomb") calorimeters. Bomb calorimeters are used to measure combustion and other gas-producing reactions, in which the reaction is observed in a strong, sealed vessel. A simple Part A In the following experiment, a coffee-cup calorimeter containing 100 mL of H20 is used. The initial temperature of the calorimeter is 23.0 °C. If 9.100 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution AHsoln of CaCl2 is -82.8 kJ/mol. constant-pressure calorimeter can be made from a foam coffee cup and a thermometer, in which energy changes in a reaction are observed via the change in temperature of the solution in the cup. The idea behind calorimeters is that if they are sufficiently insulated from the outside environment, any energy gained or lost in the chemical reaction will be directly observable as a temperature and/or pressure change in the calorimeter. Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs = 4.184 J/g .° C. Express your answer with the appropriate units. • View Available Hint(s) HẢ Value Units Submit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 16QAP: Ethyl alcohol, C2H5OH, is the intoxicating agent in liquor. Burning 1.00 g of ethyl alcohol in an...
Related questions
Question
100%
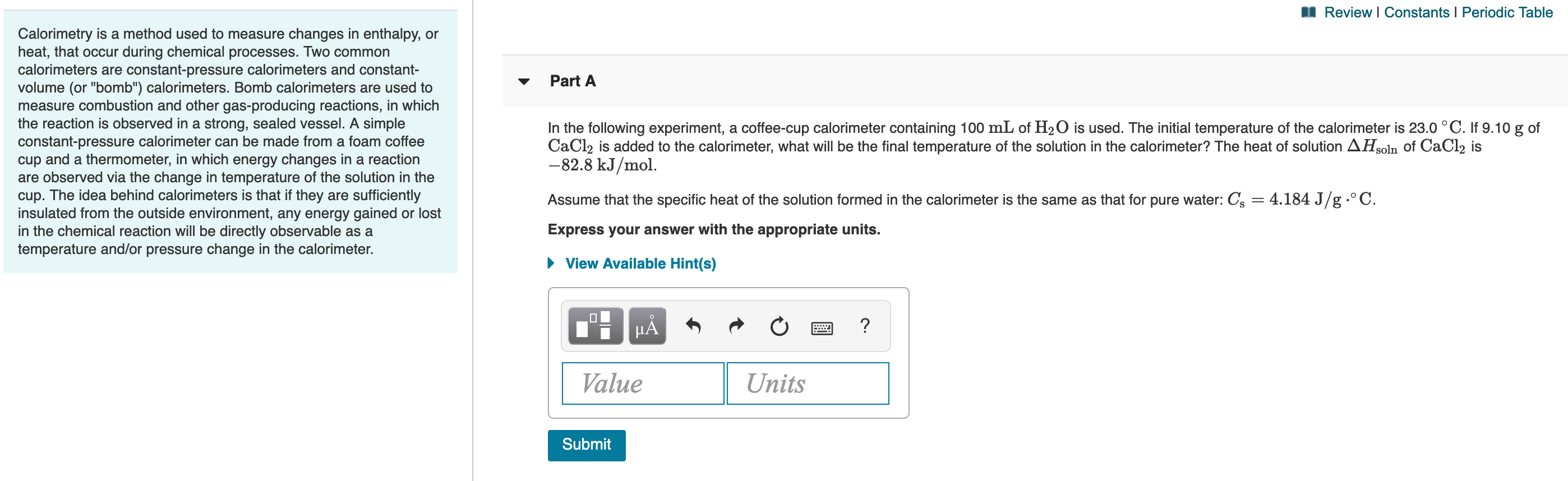
Transcribed Image Text:Review I Constants I Periodic Table
Calorimetry is a method used to measure changes in enthalpy, or
heat, that occur during chemical processes. Two common
calorimeters are constant-pressure calorimeters and constant-
volume (or "bomb") calorimeters. Bomb calorimeters are used to
measure combustion and other gas-producing reactions, in which
the reaction is observed in a strong, sealed vessel. A simple
Part A
In the following experiment, a coffee-cup calorimeter containing 100 mL of H20 is used. The initial temperature of the calorimeter is 23.0 °C. If 9.100 g of
CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution AHsoln of CaCl2 is
-82.8 kJ/mol.
constant-pressure calorimeter can be made from a foam coffee
cup and a thermometer, in which energy changes in a reaction
are observed via the change in temperature of the solution in the
cup. The idea behind calorimeters is that if they are sufficiently
insulated from the outside environment, any energy gained or lost
in the chemical reaction will be directly observable as a
temperature and/or pressure change in the calorimeter.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs = 4.184 J/g .° C.
Express your answer with the appropriate units.
• View Available Hint(s)
HẢ
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning