Review I Constants Periodic Table For every 10 m below the water surface, the pressure exerted by the water adds 14.7 psi to atmospheric pressure, so 10 m below the surface would have a total pressure of 29.4 psi. Part A You may want to reference (Pages 272 - 279) Section 7.2 while completing this problem. If a swimmer has a lung volume of 6 L at sea level, what would the volume of her lungs be when she is at the bottom of a pool that is 5.0 m deep? Assume that the temperature and amount of the air in the lungs remain unchanged. Express your answer to two significant figures and include the appropriate units. 7 НА ? Value Units Submit Request Answer P Pearson 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy Permissions | Contact Us Copyright TALL DOtrvey ToTTI TCTIOETS Only 2 left in stock - order soon. Only 10 left in stock - order 30,639 ОСТ 28 g0 F3 FI FIO F9 F8 F5 F6 F7 F4 * HAR DO
Review I Constants Periodic Table For every 10 m below the water surface, the pressure exerted by the water adds 14.7 psi to atmospheric pressure, so 10 m below the surface would have a total pressure of 29.4 psi. Part A You may want to reference (Pages 272 - 279) Section 7.2 while completing this problem. If a swimmer has a lung volume of 6 L at sea level, what would the volume of her lungs be when she is at the bottom of a pool that is 5.0 m deep? Assume that the temperature and amount of the air in the lungs remain unchanged. Express your answer to two significant figures and include the appropriate units. 7 НА ? Value Units Submit Request Answer P Pearson 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy Permissions | Contact Us Copyright TALL DOtrvey ToTTI TCTIOETS Only 2 left in stock - order soon. Only 10 left in stock - order 30,639 ОСТ 28 g0 F3 FI FIO F9 F8 F5 F6 F7 F4 * HAR DO
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.60PAE: 60 Automakers are always investigating reactions for the generation of gas to inflate air bags, in...
Related questions
Question
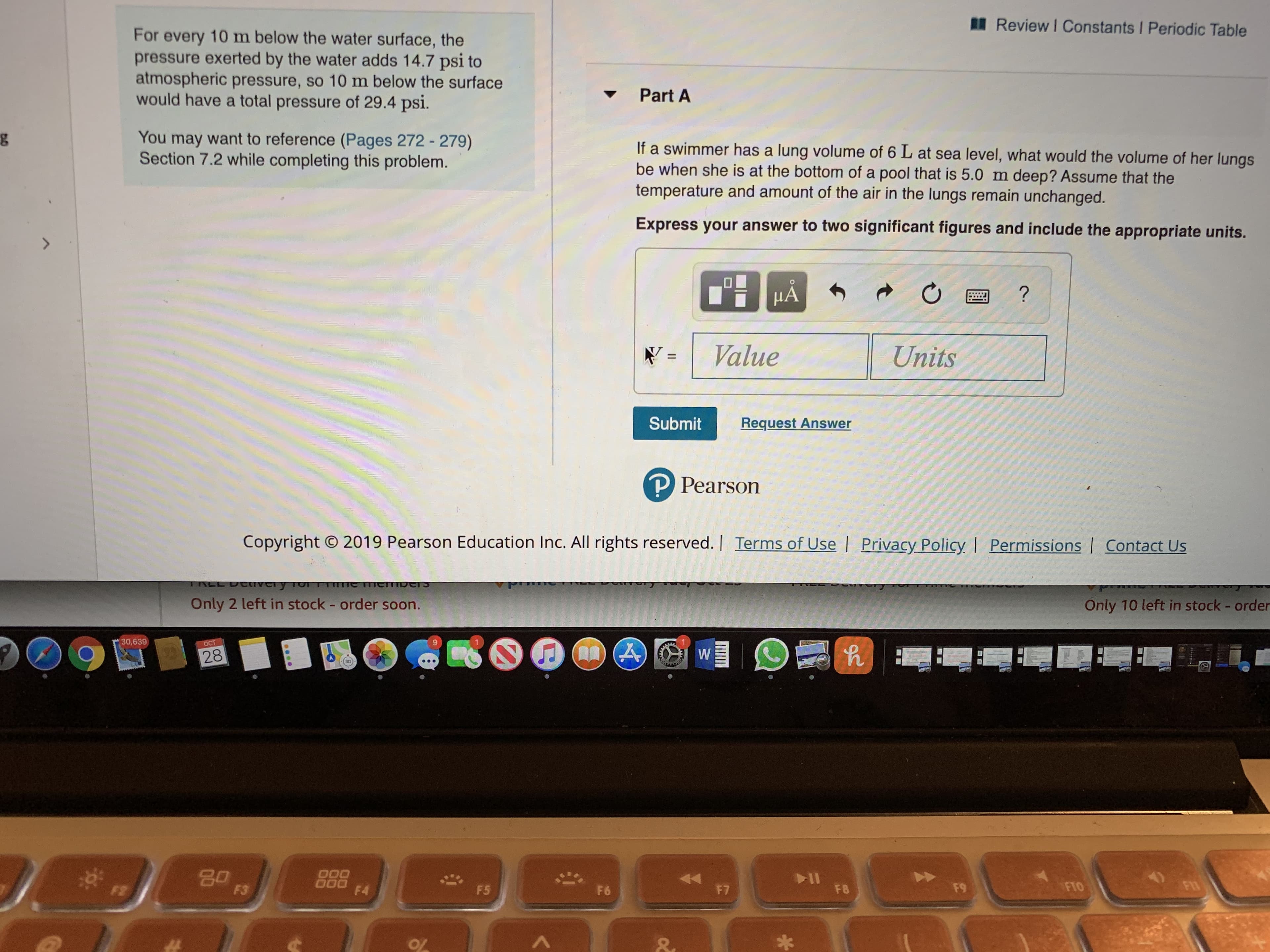
Transcribed Image Text:Review I Constants Periodic Table
For every 10 m below the water surface, the
pressure exerted by the water adds 14.7 psi to
atmospheric pressure, so 10 m below the surface
would have a total pressure of 29.4 psi.
Part A
You may
want to reference (Pages 272 - 279)
Section 7.2 while completing this problem.
If a swimmer has a lung volume of 6 L at sea level, what would the volume of her lungs
be when she is at the bottom of a pool that is 5.0 m deep? Assume that the
temperature and amount of the air in the lungs remain unchanged.
Express your answer to two significant figures and include the appropriate units.
7
НА
?
Value
Units
Submit
Request Answer
P Pearson
2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Policy Permissions | Contact Us
Copyright
TALL DOtrvey ToTTI
TCTIOETS
Only 2 left in stock - order soon.
Only 10 left in stock - order
30,639
ОСТ
28
g0
F3
FI
FIO
F9
F8
F5
F6
F7
F4
*
HAR
DO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
