Review T CO Write isotopic symbols of the form X- A (e.g. C-13) for each isotope. Part A You may want to reference (Pages 50 - 54) Section 1.8 while completing this problem. The argon isotope with 18 neutrons Enter the chemical symbol of the isotope. Request Answer Submit Part B The argon isotope with 22 neutrons Enter the chemical symbol of the isotope. Submit Request Answer
Review T CO Write isotopic symbols of the form X- A (e.g. C-13) for each isotope. Part A You may want to reference (Pages 50 - 54) Section 1.8 while completing this problem. The argon isotope with 18 neutrons Enter the chemical symbol of the isotope. Request Answer Submit Part B The argon isotope with 22 neutrons Enter the chemical symbol of the isotope. Submit Request Answer
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.8E
Related questions
Question
100%
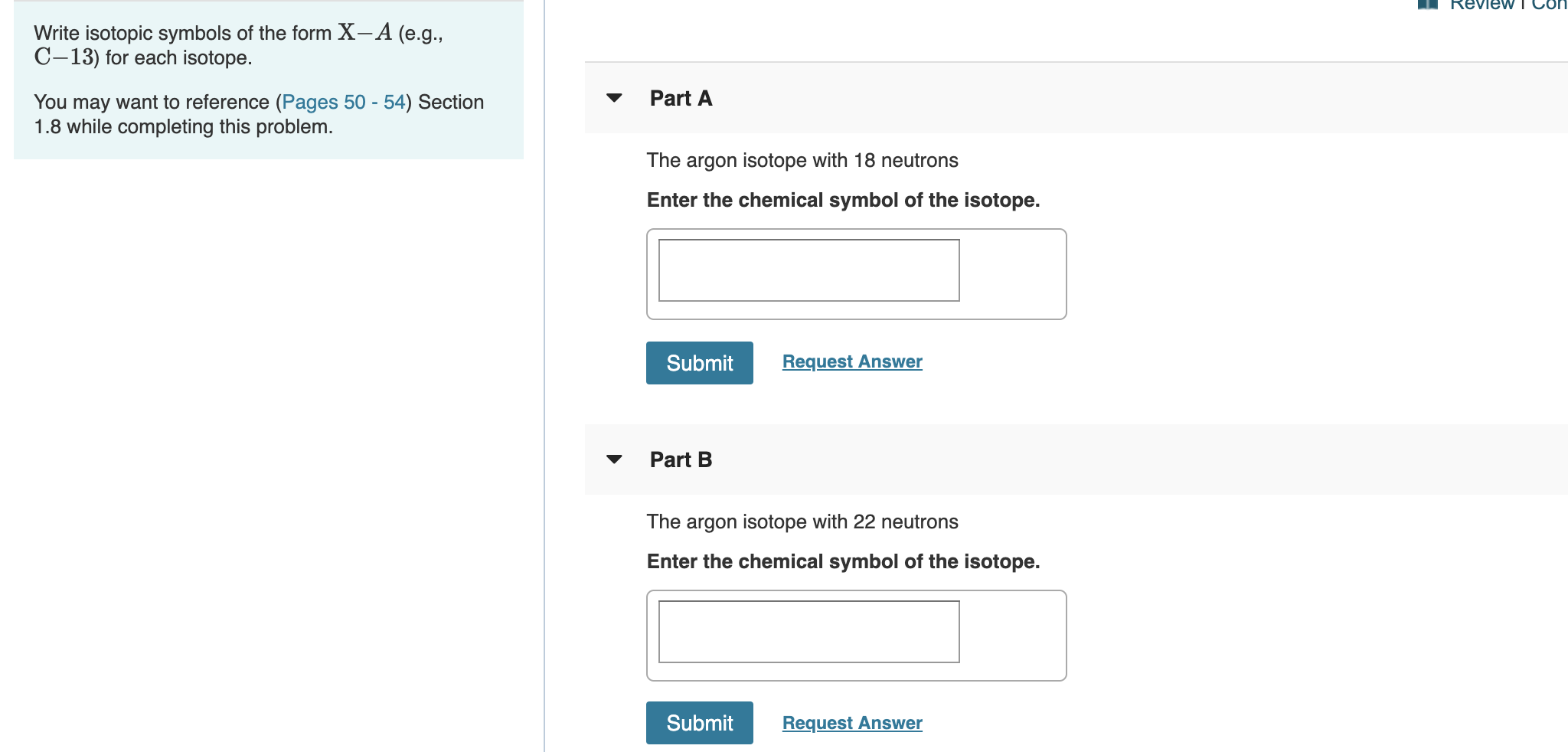
Transcribed Image Text:Review T CO
Write isotopic symbols of the form X- A (e.g.
C-13) for each isotope.
Part A
You may want to reference (Pages 50 - 54) Section
1.8 while completing this problem.
The argon isotope with 18 neutrons
Enter the chemical symbol of the isotope.
Request Answer
Submit
Part B
The argon isotope with 22 neutrons
Enter the chemical symbol of the isotope.
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning