Safari File Edit View History Bookmarks Window Help 1)) 49% Sun 10:54 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.. Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. I Review I Constants I Periodic Table In many chemical reactions of two or more compounds, ions are present in the solution that do not participate in the reaction. These ions, known as spectator ions, can be removed from the chemical equation describing the reaction. A chemical equation that has the spectator ions removed is known as a net ionic equation. Express you answer as a chemical equation including phases. • View Available Hint(s) ΑΣφ Reactions between ionic compounds often result in the precipitation of an insoluble compound. Spectator ions and precipitation products of a chemical reaction can be predicted by consulting an ionic substance solubility table. Here is one such table: Submit Compound Rule of Lit, Nat, K, or NH, Part B Always soluble What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2? NO3 or C2 H3O2 Always soluble Express you answer as a chemical equation including phases. > View Available Hint(s) Cl , Br, Insoluble with Ag+, Hg,2+, or or I Pb2+. Soluble with any other ion. Soluble with all the ions except ΑΣφ SO 2- Sr2+, Ba?+, Ca2+, Ag+, Hg22, ? or Pb2+ Soluble with Lit, Nat, K*, NH,. Insoluble with any other ion. 3 or or PO43- Soluble with Ca2+, Sr2+ , Ba?+, Lit, Nat, K+, or NH4+. Insoluble with any other ion. OH or Submit S2- Provide Feedback Next > 314150 FEB étv 23 MacBook Pro Σ esc
Safari File Edit View History Bookmarks Window Help 1)) 49% Sun 10:54 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.. Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H.. I Review I Constants I Periodic Table In many chemical reactions of two or more compounds, ions are present in the solution that do not participate in the reaction. These ions, known as spectator ions, can be removed from the chemical equation describing the reaction. A chemical equation that has the spectator ions removed is known as a net ionic equation. Express you answer as a chemical equation including phases. • View Available Hint(s) ΑΣφ Reactions between ionic compounds often result in the precipitation of an insoluble compound. Spectator ions and precipitation products of a chemical reaction can be predicted by consulting an ionic substance solubility table. Here is one such table: Submit Compound Rule of Lit, Nat, K, or NH, Part B Always soluble What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2? NO3 or C2 H3O2 Always soluble Express you answer as a chemical equation including phases. > View Available Hint(s) Cl , Br, Insoluble with Ag+, Hg,2+, or or I Pb2+. Soluble with any other ion. Soluble with all the ions except ΑΣφ SO 2- Sr2+, Ba?+, Ca2+, Ag+, Hg22, ? or Pb2+ Soluble with Lit, Nat, K*, NH,. Insoluble with any other ion. 3 or or PO43- Soluble with Ca2+, Sr2+ , Ba?+, Lit, Nat, K+, or NH4+. Insoluble with any other ion. OH or Submit S2- Provide Feedback Next > 314150 FEB étv 23 MacBook Pro Σ esc
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![Safari
File
Edit
View
History Bookmarks Window Help
1)) 49%
Sun 10:54 AM
A session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111..
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Net lonic Equations
11 of 24
<.
I Review I Constants I Periodic Table
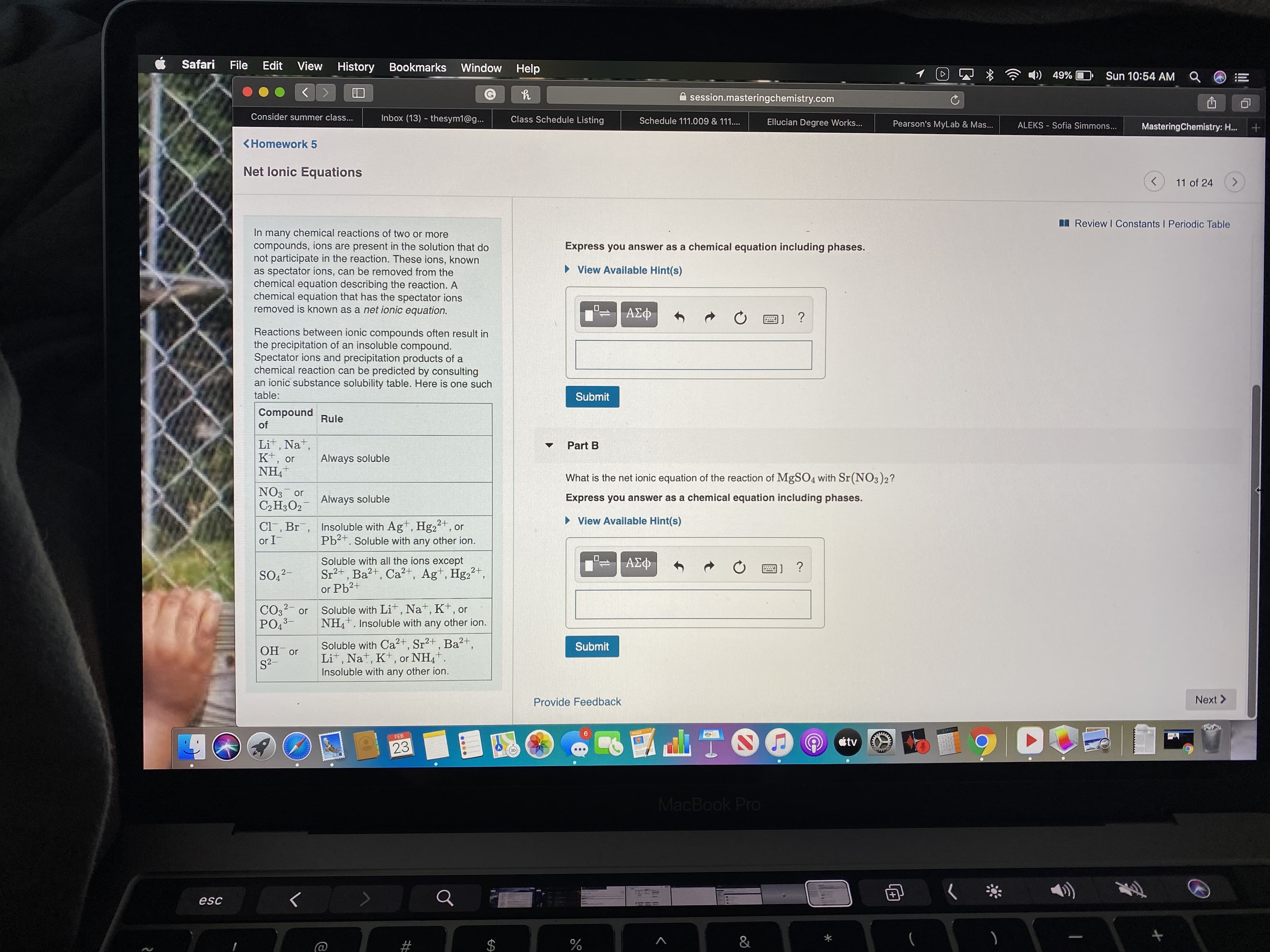
In many chemical reactions of two or more
compounds, ions are present in the solution that do
not participate in the reaction. These ions, known
as spectator ions, can be removed from the
chemical equation describing the reaction. A
chemical equation that has the spectator ions
removed is known as a net ionic equation.
Entering chemical equations
When entering chemical equations, please follow the following formatting guidelines
• Add phases to all chemical reactions unless asked otherwise. When adding phases, make sure not to place them as a subscript.
• Do not stack charges and subscripts. To add a charge to an ion with a subscript, first enter the subscript. For example, to type SO42 start by
typing S04 Then use the arrow key to arrow over before adding a superscript with the charge.
Reactions between ionic compounds often result in
the precipitation of an insoluble compound.
Spectator ions and precipitation products of a
chemical reaction can be predicted by consulting
an ionic substance solubility table. Here is one such
If you make a formatting error, you will see an message such as "Check your placement of subscripts and superscripts." or "There is an error in your
submission. Make sure you have formatted it properly." You will not lose any credit, but the answer will need to be edited and re-submitted.
table:
Part A
Compound
Rule
of
What is the net ionic equation of the reaction of ZnCl2 with NaOH?
Lit, Nat,
Kt,
NH,+
or
Always soluble
Express you answer as a chemical equation including phases.
• View Available Hint(s)
NO3 or
С2Н3О2
Always soluble
Cl, Br, Insoluble with Ag+, Hg22+, or
or I-
ΑΣΦ
1] ?
Pb2+. Soluble with any other ion.
Soluble with all the ions except
Sr2+, Ba?+, Ca?t, Ag+, Hg2",
or Pb2+
SO,2-
CO3?- or
PO3-
Soluble with Lit , Nat, K+, or
NH4. Insoluble with any other ion.
Submit
OH or
S2-
Soluble with Ca2+, Sr2+ , Ba²+,
Lit, Nat, K+, or NH4+.
Insoluble with any other ion.
Part B
What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2?
Express you answer as a chemical equation including phases.
314150
étv
FEB
23
MacBook Pro
esc](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd5c220de-a268-4902-97b2-34cd071fd5a7%2F770529b6-8337-4b0d-8018-05eacbcb3798%2Fl8q6haq.jpeg&w=3840&q=75)
Transcribed Image Text:Safari
File
Edit
View
History Bookmarks Window Help
1)) 49%
Sun 10:54 AM
A session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111..
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Net lonic Equations
11 of 24
<.
I Review I Constants I Periodic Table
In many chemical reactions of two or more
compounds, ions are present in the solution that do
not participate in the reaction. These ions, known
as spectator ions, can be removed from the
chemical equation describing the reaction. A
chemical equation that has the spectator ions
removed is known as a net ionic equation.
Entering chemical equations
When entering chemical equations, please follow the following formatting guidelines
• Add phases to all chemical reactions unless asked otherwise. When adding phases, make sure not to place them as a subscript.
• Do not stack charges and subscripts. To add a charge to an ion with a subscript, first enter the subscript. For example, to type SO42 start by
typing S04 Then use the arrow key to arrow over before adding a superscript with the charge.
Reactions between ionic compounds often result in
the precipitation of an insoluble compound.
Spectator ions and precipitation products of a
chemical reaction can be predicted by consulting
an ionic substance solubility table. Here is one such
If you make a formatting error, you will see an message such as "Check your placement of subscripts and superscripts." or "There is an error in your
submission. Make sure you have formatted it properly." You will not lose any credit, but the answer will need to be edited and re-submitted.
table:
Part A
Compound
Rule
of
What is the net ionic equation of the reaction of ZnCl2 with NaOH?
Lit, Nat,
Kt,
NH,+
or
Always soluble
Express you answer as a chemical equation including phases.
• View Available Hint(s)
NO3 or
С2Н3О2
Always soluble
Cl, Br, Insoluble with Ag+, Hg22+, or
or I-
ΑΣΦ
1] ?
Pb2+. Soluble with any other ion.
Soluble with all the ions except
Sr2+, Ba?+, Ca?t, Ag+, Hg2",
or Pb2+
SO,2-
CO3?- or
PO3-
Soluble with Lit , Nat, K+, or
NH4. Insoluble with any other ion.
Submit
OH or
S2-
Soluble with Ca2+, Sr2+ , Ba²+,
Lit, Nat, K+, or NH4+.
Insoluble with any other ion.
Part B
What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2?
Express you answer as a chemical equation including phases.
314150
étv
FEB
23
MacBook Pro
esc
Transcribed Image Text:Safari
File
Edit
View
History Bookmarks
Window Help
) 49%
Sun 10:54 AM
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g...
Class Schedule Listing
Schedule 111.009 & 111..
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H..
<Homework 5
Net lonic Equations
11 of 24
<>
I Review I Constants I Periodic Table
In many chemical reactions of two or more
compounds, ions are present in the solution that do
not participate in the reaction. These ions, known
as spectator ions, can be removed from the
chemical equation describing the reaction. A
chemical equation that has the spectator ions
removed is known as a net ionic equation.
Express you answer as a chemical equation including phases.
• View Available Hint(s)
ΑΣφ
Reactions between ionic compounds often result in
the precipitation of an insoluble compound.
Spectator ions and precipitation products of a
chemical reaction can be predicted by consulting
an ionic substance solubility table. Here is one such
table:
Submit
Compound
Rule
of
Lit, Nat,
K, or
NH,
Part B
Always soluble
What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2?
NO3 or
C2 H3O2
Always soluble
Express you answer as a chemical equation including phases.
> View Available Hint(s)
Cl , Br, Insoluble with Ag+, Hg,2+, or
or I
Pb2+. Soluble with any other ion.
Soluble with all the ions except
ΑΣφ
SO 2-
Sr2+, Ba?+, Ca2+, Ag+, Hg22,
?
or Pb2+
Soluble with Lit, Nat, K*,
NH,. Insoluble with any other ion.
3 or
or
PO43-
Soluble with Ca2+, Sr2+ , Ba?+,
Lit, Nat, K+, or NH4+.
Insoluble with any other ion.
OH or
Submit
S2-
Provide Feedback
Next >
314150
FEB
étv
23
MacBook Pro
Σ
esc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY