Safari File Edit View History Bookmarks Window Help )) 48% Sun 10:59 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g.. Class Schedule Listing Schedule 111.009 & 111.... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H...
Safari File Edit View History Bookmarks Window Help )) 48% Sun 10:59 AM session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g.. Class Schedule Listing Schedule 111.009 & 111.... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H...
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Safari
File
Edit View History Bookmarks
Window Help
)) 48%
Sun 10:59 AM
session.masteringchemistry.com
Consider summer class...
Inbox (13) - thesym1@g..
Class Schedule Listing
Schedule 111.009 & 111....
Ellucian Degree Works...
Pearson's MyLab & Mas...
ALEKS - Sofia Simmons...
MasteringChemistry: H...
<Homework 5
Acids, Bases, and Salts
17 of 24
<.
I Review I Constants I Periodic Table
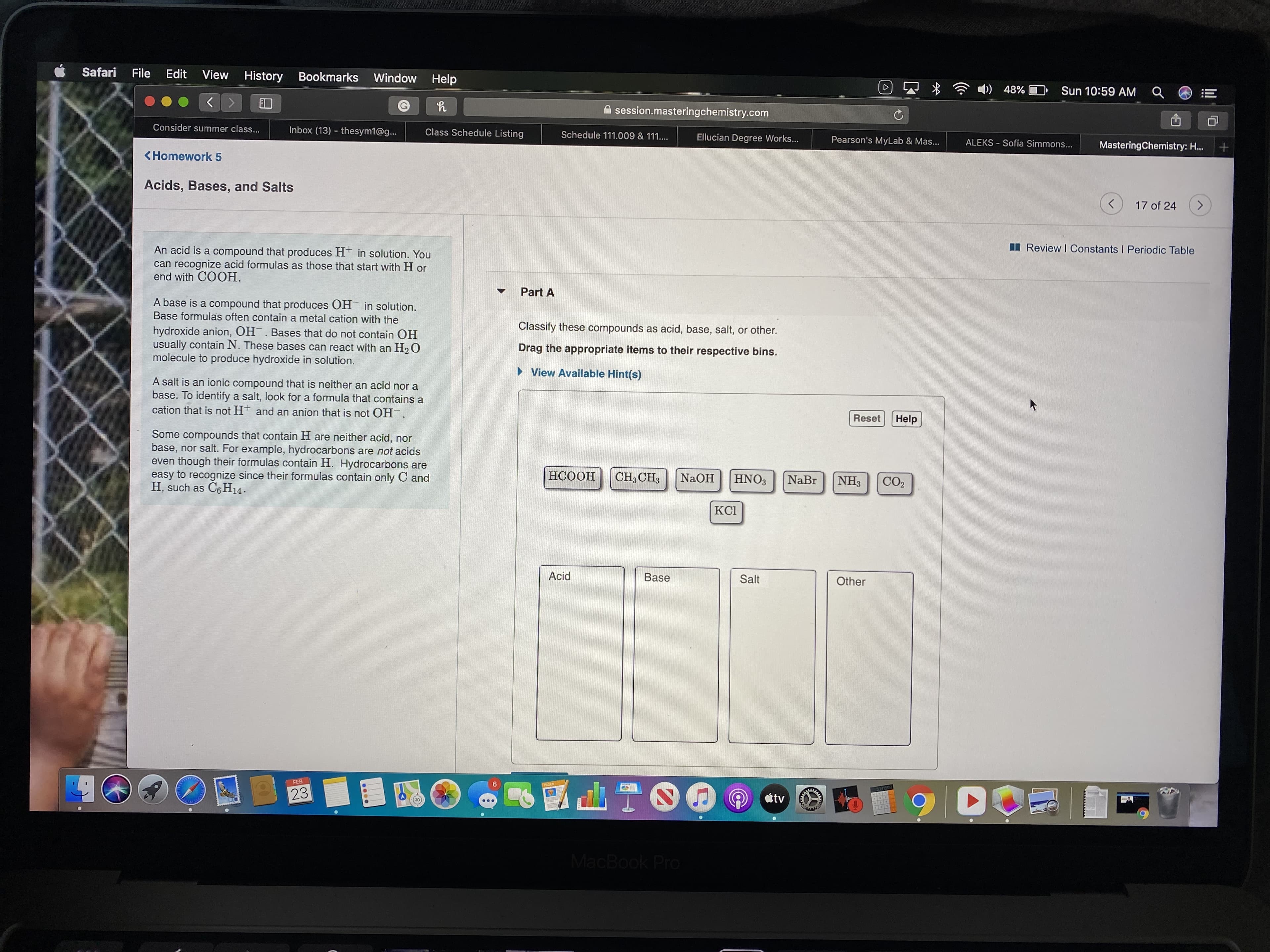
An acid is a compound that produces H in solution. You
can recognize acid formulas as those that start with H or
end with COOH.
Part A
A base is a compound that produces OH in solution.
Base formulas often contain a metal cation with the
Classify these compounds as acid, base, salt, or other.
hydroxide anion, OH. Bases that do not contain OH
usually contain N. These bases can react with an H2 O
molecule to produce hydroxide in solution.
Drag the appropriate items to their respective bins.
• View Available Hint(s)
A salt is an ionic compound that is neither an acid nor a
base. To identify a salt, look for a formula that contains a
cation that is not H and an anion that is not OH.
Reset
Help
Some compounds that contain H are neither acid, nor
base, nor salt. For example, hydrocarbons are not acids
even though their formulas contain H. Hydrocarbons are
easy to recognize since their formulas contain only C and
H, such as C6 H14.
НСООН
CH3 CH3
NaOH
HNO3
NaBr
NH3
CO2
КСІ
Acid
Base
Salt
Other
FEB
PACED
3.141503
23
tv
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

