Sapling Learning | Lenoir-Rhyne | X y! Yahoo - login acid or base calcul 17.3: Acid-Base Tit y! Calculate the pH o calculator - Googe b A 0.760g0.760 gsa https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017 Sapling Learning HW 14A Homeworki Leticia Venancio macmillan learning Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations E 3 of 13 Questions く O Assignment Score: Ex Give Up? O Hint 73.4% Resources Check Answer 1 Question 0% Question 3 of 13 Attempt 2 O of o Attempts Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCl(aq) is added to 45.0 mL of 0.350 M NaOH(aq). 2 Question 0% O of o Attempts pH : 0.93 3 Question 0% 1 of o Attempts In Progress Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCI(aq) is added to 25.0 mL of 0.450 M NaOH(aq). O4 Question 100% 1 of o Attempts pH : 3.21 Correct O 5 Question 99.3% 3 of o Attempts Correct O 6 Question 100% 1 of o Attempts Correct O7 Question 100% 1 of o Attempts Correct O8 Question 99% 2 of o Attempts Correct 9 Question 60% 1 of o Attempts In Progress Question Source: MRG - General Chemistry | Publisher: University Science Books © 2011-2020 Sapling Learning, Inc. about us privacy policy terms of use contact us help careers 3:24 PM O Type here to search へ 23 4/5/2020 K入 KY
Sapling Learning | Lenoir-Rhyne | X y! Yahoo - login acid or base calcul 17.3: Acid-Base Tit y! Calculate the pH o calculator - Googe b A 0.760g0.760 gsa https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017 Sapling Learning HW 14A Homeworki Leticia Venancio macmillan learning Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations E 3 of 13 Questions く O Assignment Score: Ex Give Up? O Hint 73.4% Resources Check Answer 1 Question 0% Question 3 of 13 Attempt 2 O of o Attempts Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCl(aq) is added to 45.0 mL of 0.350 M NaOH(aq). 2 Question 0% O of o Attempts pH : 0.93 3 Question 0% 1 of o Attempts In Progress Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCI(aq) is added to 25.0 mL of 0.450 M NaOH(aq). O4 Question 100% 1 of o Attempts pH : 3.21 Correct O 5 Question 99.3% 3 of o Attempts Correct O 6 Question 100% 1 of o Attempts Correct O7 Question 100% 1 of o Attempts Correct O8 Question 99% 2 of o Attempts Correct 9 Question 60% 1 of o Attempts In Progress Question Source: MRG - General Chemistry | Publisher: University Science Books © 2011-2020 Sapling Learning, Inc. about us privacy policy terms of use contact us help careers 3:24 PM O Type here to search へ 23 4/5/2020 K入 KY
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 4P
Related questions
Question
Transcribed Image Text:Sapling Learning |
Lenoir-Rhyne | X
y! Yahoo - login
acid or base calcul
17.3: Acid-Base Tit
y! Calculate the pH o
calculator - Googe b A 0.760g0.760 gsa
https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017
Sapling Learning
HW 14A Homeworki
Leticia Venancio
macmillan learning
Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations
E 3 of 13 Questions
く
O Assignment Score:
Ex Give Up?
O Hint
73.4%
Resources
Check Answer
1 Question
0%
Question 3 of 13
Attempt 2
O of o Attempts
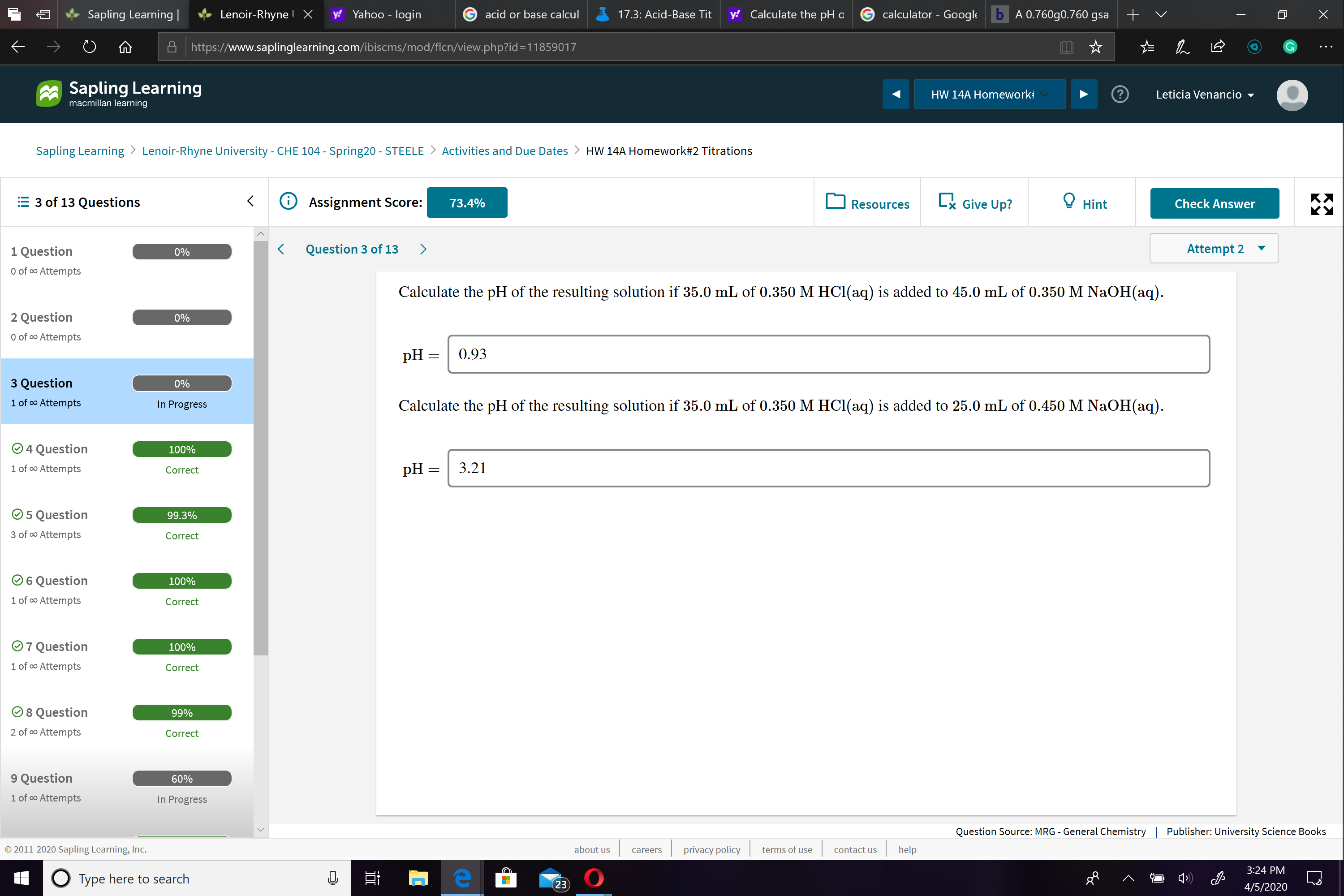
Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCl(aq) is added to 45.0 mL of 0.350 M NaOH(aq).
2 Question
0%
O of o Attempts
pH :
0.93
3 Question
0%
1 of o Attempts
In Progress
Calculate the pH of the resulting solution if 35.0 mL of 0.350 M HCI(aq) is added to 25.0 mL of 0.450 M NaOH(aq).
O4 Question
100%
1 of o Attempts
pH :
3.21
Correct
O 5 Question
99.3%
3 of o Attempts
Correct
O 6 Question
100%
1 of o Attempts
Correct
O7 Question
100%
1 of o Attempts
Correct
O8 Question
99%
2 of o Attempts
Correct
9 Question
60%
1 of o Attempts
In Progress
Question Source: MRG - General Chemistry | Publisher: University Science Books
© 2011-2020 Sapling Learning, Inc.
about us
privacy policy
terms of use
contact us
help
careers
3:24 PM
O Type here to search
へ
23
4/5/2020
K入
KY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning