Select the correct total ionic equation for the reaction of iron(III) nitrate with sodium hydroxide. Read each answer choice carefully. Fe(NO3)з (aq) + 3NaOH (аq) — Fe(ОН)з (s) + 3NaNO3 (аq) Fest (aq) + 3NO3° (aq) + 3Na* (aq) + 30H¯ (aq) → Fe(OH)3 (s) + 3Na* (aq) + 3NO3 (aq) + NO3 (aq) + Na* (aq) → NaNO3 (s) Fe(NO3)3 (aq) + 3NAOH (aq) → Fe(OH)3 (aq) + 3NaNO3 (s) Fe3+ (aд) + 3ОН (аq) — Fe(ОH)з (s) Fe3+ (aд) + 3NO3 (аq) + 3Na* (аq) + 3он (ад) — Fe$* (aq) + 3ОН (аq)+ 3NaNO3 (s)
Select the correct total ionic equation for the reaction of iron(III) nitrate with sodium hydroxide. Read each answer choice carefully. Fe(NO3)з (aq) + 3NaOH (аq) — Fe(ОН)з (s) + 3NaNO3 (аq) Fest (aq) + 3NO3° (aq) + 3Na* (aq) + 30H¯ (aq) → Fe(OH)3 (s) + 3Na* (aq) + 3NO3 (aq) + NO3 (aq) + Na* (aq) → NaNO3 (s) Fe(NO3)3 (aq) + 3NAOH (aq) → Fe(OH)3 (aq) + 3NaNO3 (s) Fe3+ (aд) + 3ОН (аq) — Fe(ОH)з (s) Fe3+ (aд) + 3NO3 (аq) + 3Na* (аq) + 3он (ад) — Fe$* (aq) + 3ОН (аq)+ 3NaNO3 (s)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 46PS: Balance each of the following equations, and then write the net ionic equation. Show states for all...
Related questions
Question
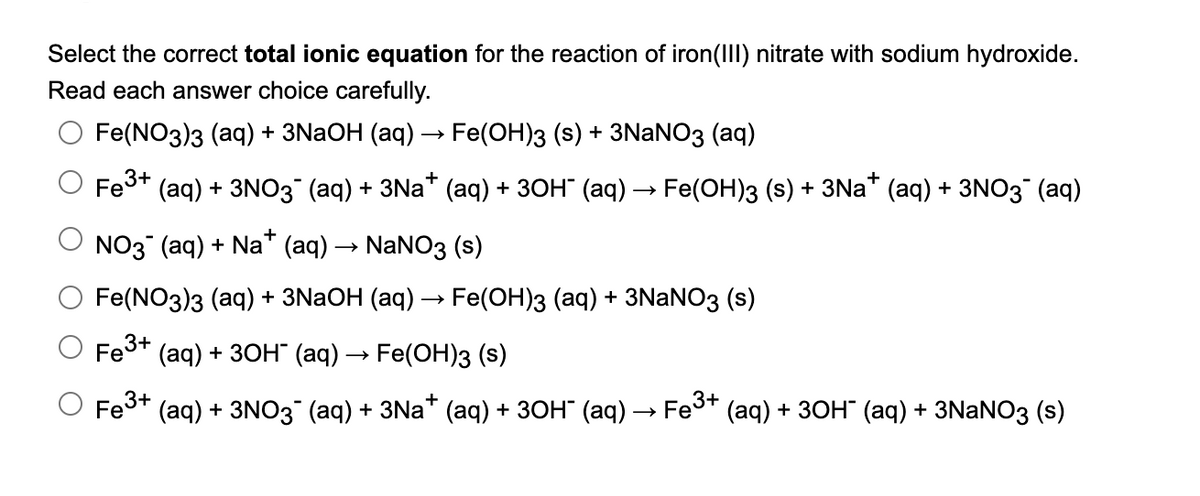
Transcribed Image Text:Select the correct total ionic equation for the reaction of iron(III) nitrate with sodium hydroxide.
Read each answer choice carefully.
Fe(NO3)з (aq) + 3NaOH (аq) — Fe(ОН)з (s) + 3NaNO3 (aq)
Fe3+
(aq) + 3NO3 (аq) + 3Na* (аq) + зон (аq) —> Fe(ОН)з (s) + 3Na* (aq) + 3NO3 (aq)
O NO3 (aq) + Na* (aq) → NaNO3 (s)
O Fe(NO3)з (аq) + 3NaOH (аq) — Fe(ОH)3 (аq) + 3NaNO3 (s)
O Fe3+ (aq) + 3он (ад) — Fe(OН)з (s)
O Fes* (aq) + 3NO3 (aq) + 3Na* (aq) + 3он (аq) — Fes* (aq) + 3он (аq) + 3NaNOз (s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning