Sigma Bonding Ac bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded muclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH 1s orbital What atomic or hybrid orbitals make up the sigma bond between C, and Cl in dichloroethylene, CH,CCI, ? (Cz is the second carbon in the structure as written.) orbital on C2 + orbital on CI What is the approximate CC-CI bond angle ?
Sigma Bonding Ac bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded muclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH 1s orbital What atomic or hybrid orbitals make up the sigma bond between C, and Cl in dichloroethylene, CH,CCI, ? (Cz is the second carbon in the structure as written.) orbital on C2 + orbital on CI What is the approximate CC-CI bond angle ?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 4E: Consider the incomplete orbital representation of O2 , below right. a. Identify which lobes are...
Related questions
Question
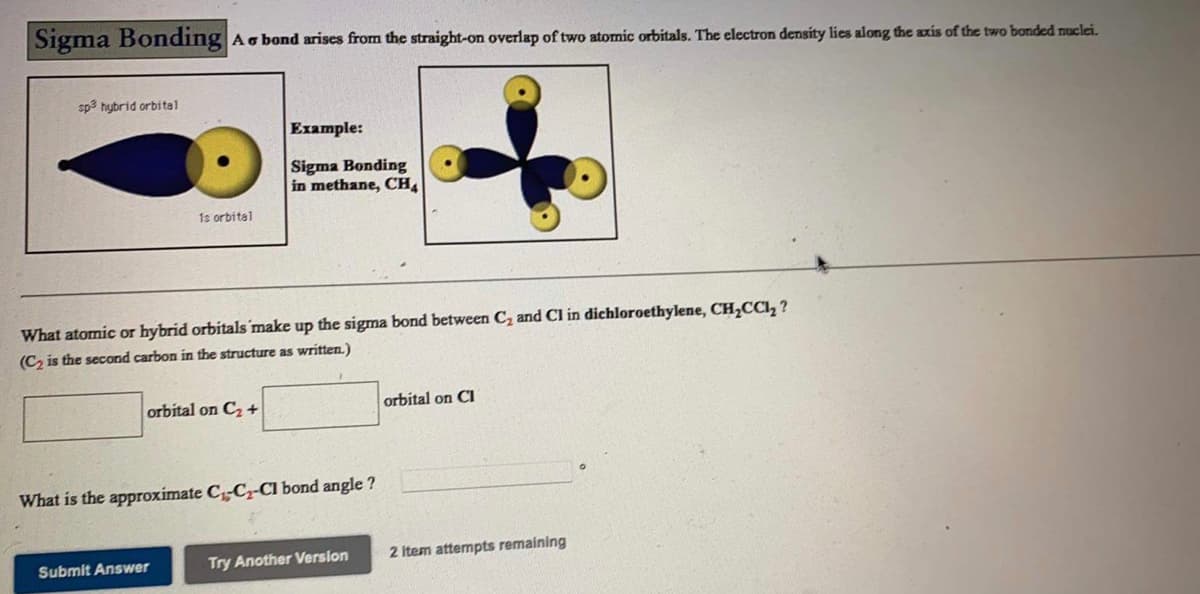
Transcribed Image Text:Sigma Bonding
Ao bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded uclei.
sp3 hybrid orbital
Example:
Sigma Bonding
in methane, CH
1s orbital
What atomic or hybrid orbitals make up the sigma bond between C, and Cl in dichloroethylene, CH,CCI, ?
(C, is the second carbon in the structure as written.)
orbital on C +
orbital on CI
What is the approximate C-C-Cl bond angle ?
Try Another Version
2 Item attempts remaining
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning