Silvocarcin M is isolated from Streptomyces strains and has strong antitumor activity. OMe OH OMe H OH OH CH3 H3C OH Gilvocarcin M Suzuki and coworkers were able to carry out the total synthesis of naturally occurring (-)-gilvocarcin M. Their synthesis included the following steps. (The wavy line means that stereochemistry is unspecified or is a mixture.) The stereochemistry of the product appears to be counterintuitive (apparent attack from the more hindered side). The rea- son is that the reaction involves initial O-alkylation followed by a rearrangement that need not concern us. OBn OBn H OBn H OBn OBn CH Lewis acid HO OBn CH AcO we НО Bn = CH,Ph OBn OBn %3! (A) (В)
Silvocarcin M is isolated from Streptomyces strains and has strong antitumor activity. OMe OH OMe H OH OH CH3 H3C OH Gilvocarcin M Suzuki and coworkers were able to carry out the total synthesis of naturally occurring (-)-gilvocarcin M. Their synthesis included the following steps. (The wavy line means that stereochemistry is unspecified or is a mixture.) The stereochemistry of the product appears to be counterintuitive (apparent attack from the more hindered side). The rea- son is that the reaction involves initial O-alkylation followed by a rearrangement that need not concern us. OBn OBn H OBn H OBn OBn CH Lewis acid HO OBn CH AcO we НО Bn = CH,Ph OBn OBn %3! (A) (В)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.37P
Related questions
Question
This reaction gives both high regioselectivity and stereoselectivity. What other products might have been expected?
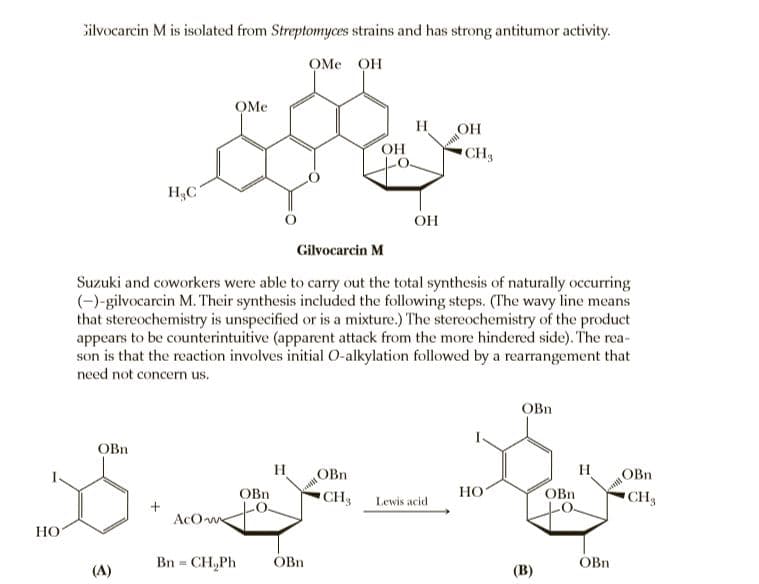
Transcribed Image Text:Silvocarcin M is isolated from Streptomyces strains and has strong antitumor activity.
OMe OH
OMe
H
OH
OH
CH3
H3C
OH
Gilvocarcin M
Suzuki and coworkers were able to carry out the total synthesis of naturally occurring
(-)-gilvocarcin M. Their synthesis included the following steps. (The wavy line means
that stereochemistry is unspecified or is a mixture.) The stereochemistry of the product
appears to be counterintuitive (apparent attack from the more hindered side). The rea-
son is that the reaction involves initial O-alkylation followed by a rearrangement that
need not concern us.
OBn
OBn
H
OBn
H
OBn
OBn
CH Lewis acid
HO
OBn
CH
AcO we
НО
Bn = CH,Ph
OBn
OBn
%3!
(A)
(В)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
