skmarks Feople Tab X 11% Chapter 14 Lecture Two o x G periodic table with charge x ourses/1885023/quizzes/4613315 Incorrect Question 1 0/4.54 pts What are the intermolecular forces? oject d A.d Forces of attraction between two different atoms bonding together via a covalent bond. Forces of attraction between two separate molecules. Forces of attraction between two ions. Forces of attraction between two atoms of the same kind that are bonded via a covalent bond. None of these are correct. 4. MacBook Pro FII F10 F9 F8 F7
skmarks Feople Tab X 11% Chapter 14 Lecture Two o x G periodic table with charge x ourses/1885023/quizzes/4613315 Incorrect Question 1 0/4.54 pts What are the intermolecular forces? oject d A.d Forces of attraction between two different atoms bonding together via a covalent bond. Forces of attraction between two separate molecules. Forces of attraction between two ions. Forces of attraction between two atoms of the same kind that are bonded via a covalent bond. None of these are correct. 4. MacBook Pro FII F10 F9 F8 F7
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 2CTQ: Figure 4.1 is a cartoon depiction of liquid water at the molecular level. a. (E) Label at least one...
Related questions
Question
Intermolecular forces. Chemistry. Help.
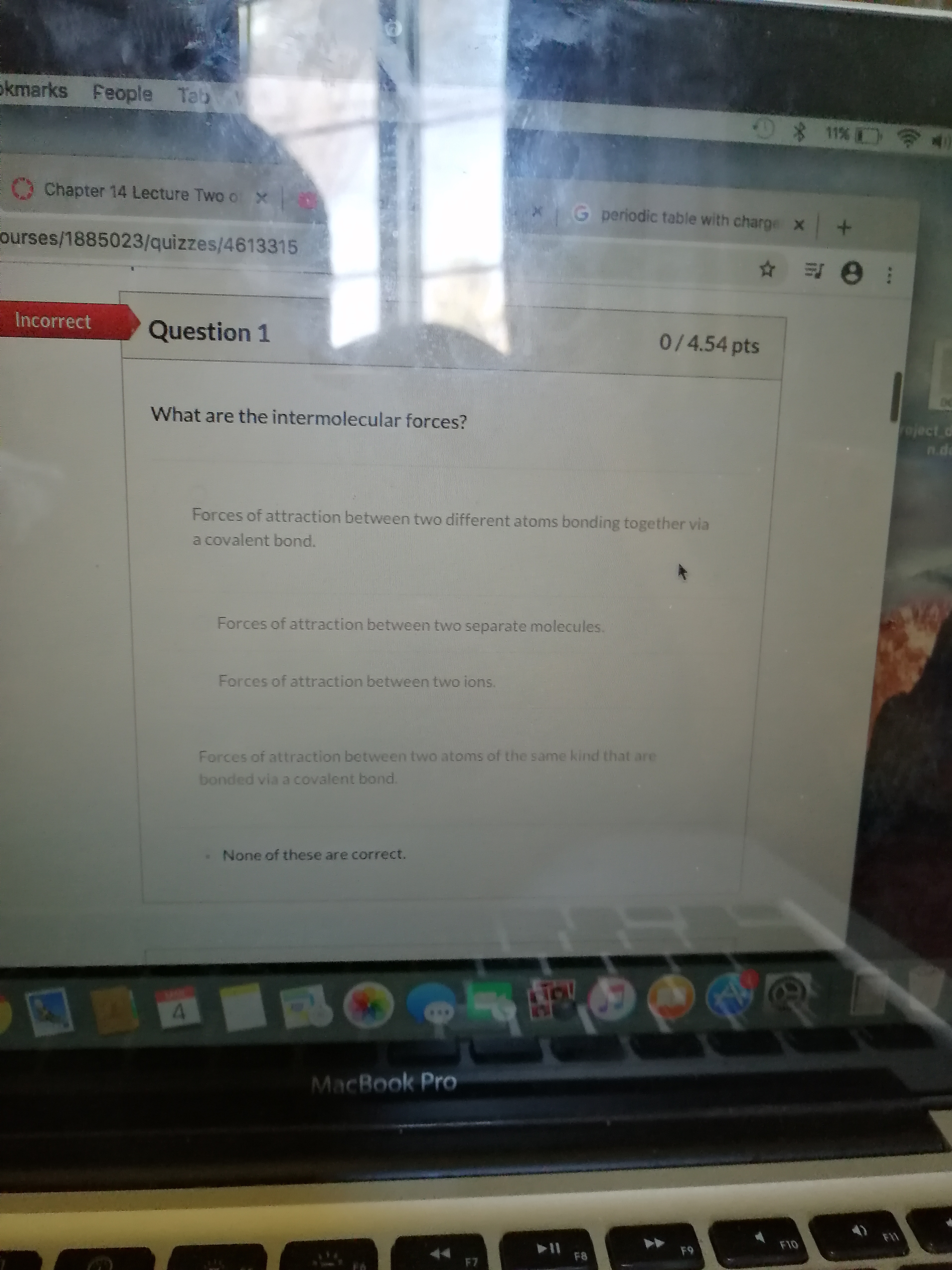
Transcribed Image Text:skmarks Feople Tab
X 11%
Chapter 14 Lecture Two o x
G periodic table with charge x
ourses/1885023/quizzes/4613315
Incorrect
Question 1
0/4.54 pts
What are the intermolecular forces?
oject d
A.d
Forces of attraction between two different atoms bonding together via
a covalent bond.
Forces of attraction between two separate molecules.
Forces of attraction between two ions.
Forces of attraction between two atoms of the same kind that are
bonded via a covalent bond.
None of these are correct.
4.
MacBook Pro
FII
F10
F9
F8
F7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
