Solving for a reactant using a chemical equation Wine goco vad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the alr to form water (H,0) and acetic acid (0,) (CH,COOH), the main ingredient of vinegar. What mass of acetic acid is produced by the reaction of 4.1 g of oxygen gas? Round your answer to 2 significant digits. x10 Chrome OS System 41m Sign-in has changed Account update required Explanation Check 2020 McGraw-Hill E DELL 11
Solving for a reactant using a chemical equation Wine goco vad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the alr to form water (H,0) and acetic acid (0,) (CH,COOH), the main ingredient of vinegar. What mass of acetic acid is produced by the reaction of 4.1 g of oxygen gas? Round your answer to 2 significant digits. x10 Chrome OS System 41m Sign-in has changed Account update required Explanation Check 2020 McGraw-Hill E DELL 11
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 25QAP: Cyclopropane mixed in the proper ratio with oxygen can be used as an anesthetic. At 755 mm Hg and...
Related questions
Question
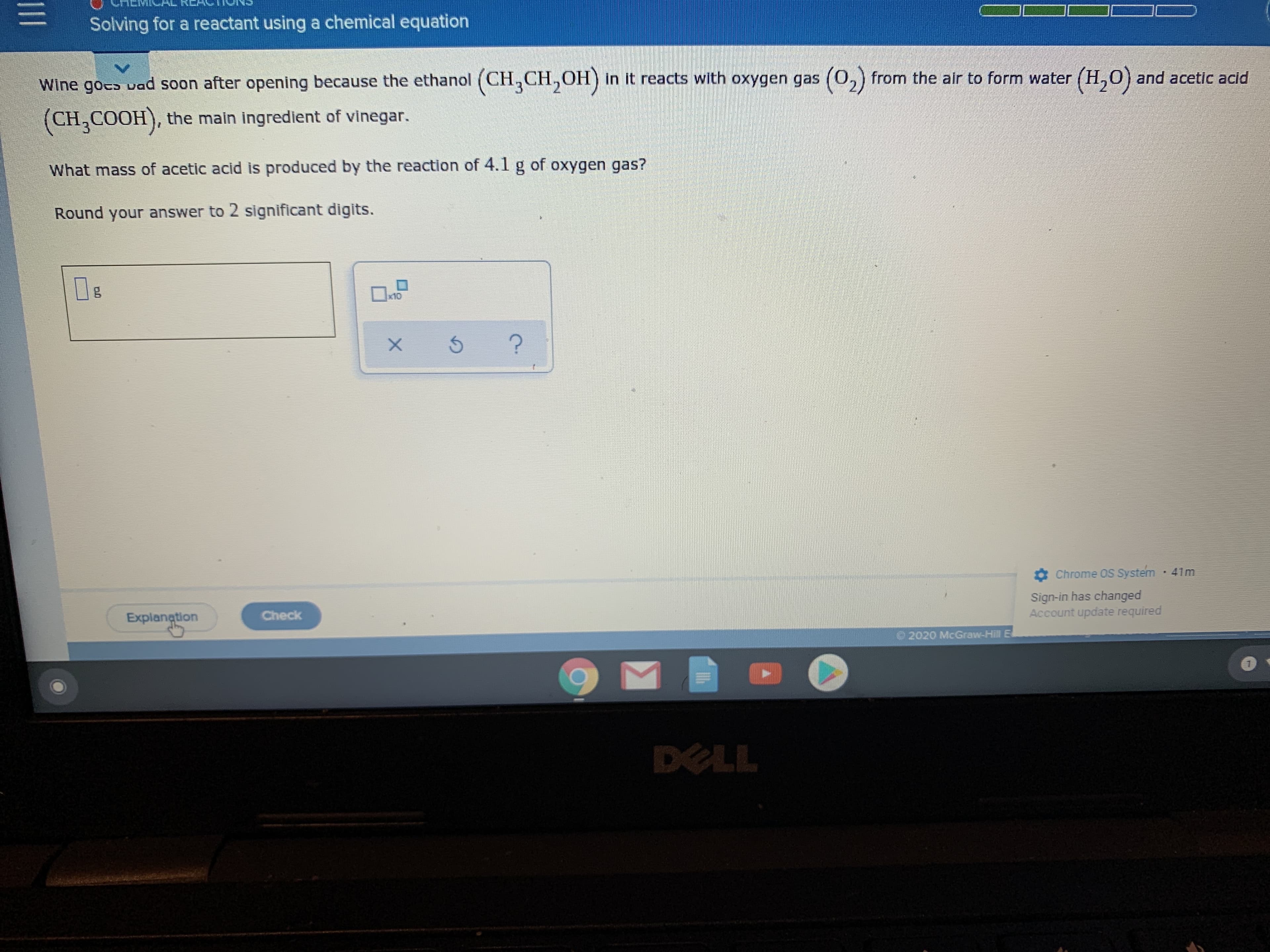
Transcribed Image Text:Solving for a reactant using a chemical equation
Wine goco vad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the alr to form water (H,0) and acetic acid
(0,)
(CH,COOH), the main ingredient of vinegar.
What mass of acetic acid is produced by the reaction of 4.1 g of oxygen gas?
Round your answer to 2 significant digits.
x10
Chrome OS System
41m
Sign-in has changed
Account update required
Explanation
Check
2020 McGraw-Hill E
DELL
11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
