Specific Heat Practice Problems Use the table below to answer the following questions. Identify all variables and show all of your work with units. Useful information: q= Cp X m x AT Specific Heat (J/g•°C) Substance cp Specific heat See values > 4.179 water heat energy Joules (J) 0.900 aluminum + means energy gained means energy lost 0.385 copper т Grams (g) iron 0.450 mass 4T Change in temp Tr-Ti (°C) granite 0.790 1. When 3.0 kg of water is cooled from 80.0°C to 10.0°C, how much heat energy is losť? Answer Knowns and unknown (?) Plug values into formula Cp= 4,18 q= 4T= 2. How much heat is needed to raise a 0.30 kg piece of aluminum from 30.°C to 150°C? Answer Knowns and unknown (?) Plug values into formula q= Cp= AT= 3. Calculate the temperature change when: a) 10.0 kg of water loses 232 kJ of heat. Solve for unknown Answer Knowns and unknown (?) Plug values into formula Cp= =b AT= m= b) 1.96 kJ of heat are added to 500. g of copper. Plug values into formula Solve for unknown Answer Knowns and unknown (?) Cp= AT= *when heated, the temperature of a water sample increased from 15°C to 39°C. It absorbed 4300 joules of heat. What is the mass of the sample? 3. 3.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? 0. The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release of 47 Joules of heat. What is the specific heat of iron? 7. The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 Joules of heat. What is the mass of the sample? 8. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? 9. If a 3.1g ring is heated using 10.0 J, its temperature rises 17.9°C. Calculate the specific heat capacity of the ring. 10. What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its temperature changes from 25°C to 20°C?
Specific Heat Practice Problems Use the table below to answer the following questions. Identify all variables and show all of your work with units. Useful information: q= Cp X m x AT Specific Heat (J/g•°C) Substance cp Specific heat See values > 4.179 water heat energy Joules (J) 0.900 aluminum + means energy gained means energy lost 0.385 copper т Grams (g) iron 0.450 mass 4T Change in temp Tr-Ti (°C) granite 0.790 1. When 3.0 kg of water is cooled from 80.0°C to 10.0°C, how much heat energy is losť? Answer Knowns and unknown (?) Plug values into formula Cp= 4,18 q= 4T= 2. How much heat is needed to raise a 0.30 kg piece of aluminum from 30.°C to 150°C? Answer Knowns and unknown (?) Plug values into formula q= Cp= AT= 3. Calculate the temperature change when: a) 10.0 kg of water loses 232 kJ of heat. Solve for unknown Answer Knowns and unknown (?) Plug values into formula Cp= =b AT= m= b) 1.96 kJ of heat are added to 500. g of copper. Plug values into formula Solve for unknown Answer Knowns and unknown (?) Cp= AT= *when heated, the temperature of a water sample increased from 15°C to 39°C. It absorbed 4300 joules of heat. What is the mass of the sample? 3. 3.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? 0. The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release of 47 Joules of heat. What is the specific heat of iron? 7. The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 Joules of heat. What is the mass of the sample? 8. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? 9. If a 3.1g ring is heated using 10.0 J, its temperature rises 17.9°C. Calculate the specific heat capacity of the ring. 10. What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its temperature changes from 25°C to 20°C?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.104PAE: 9.104 An engineer is using sodium metal as a cooling agent in a design because it has useful thermal...
Related questions
Question
100%
Can you help me, im confused on how to do this So can you help me understand it by showing how to answer and set up number 1 - 6?
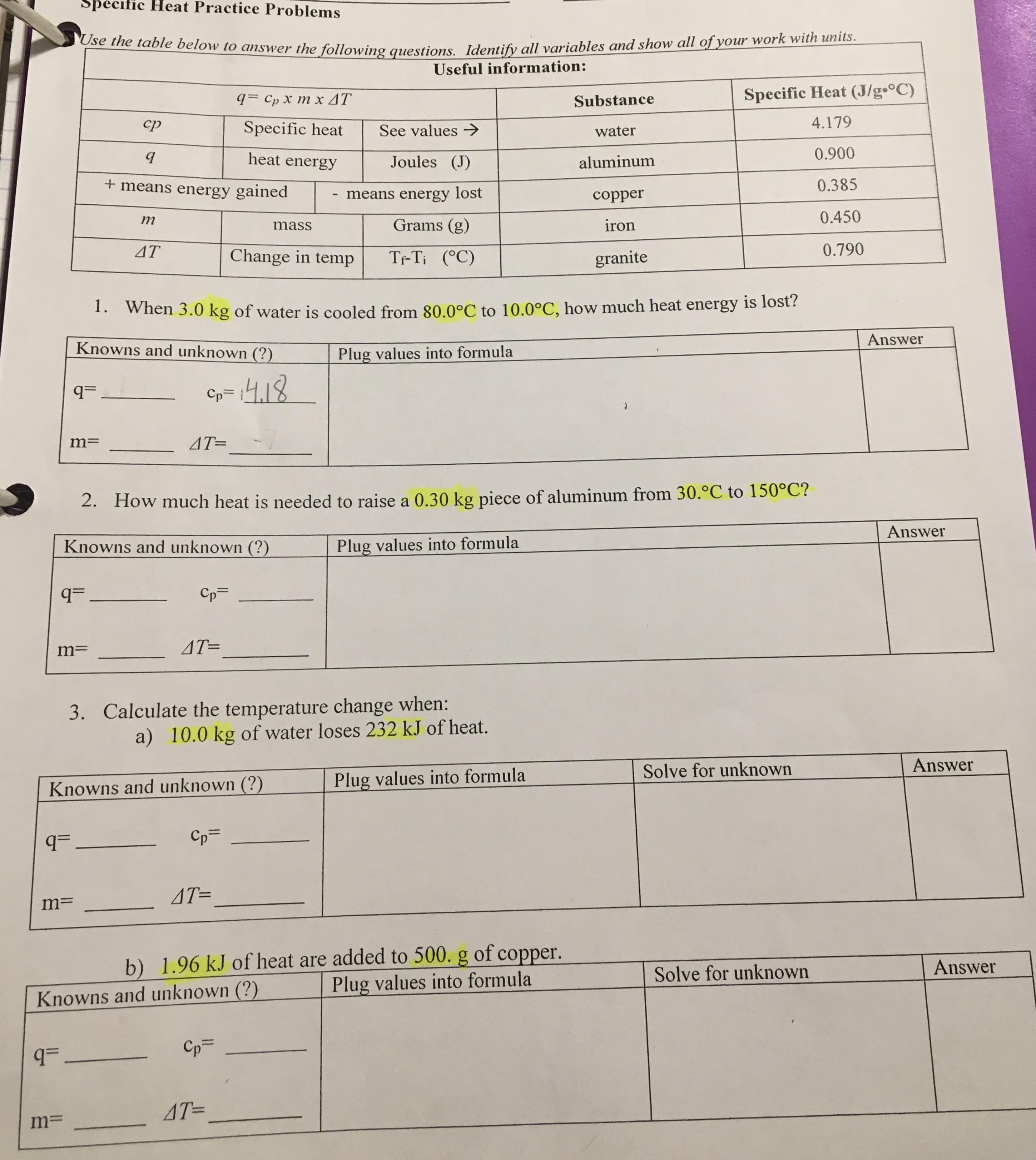
Transcribed Image Text:Specific Heat Practice Problems
Use the table below to answer the following questions. Identify all variables and show all of your work with units.
Useful information:
q= Cp X m x AT
Specific Heat (J/g•°C)
Substance
cp
Specific heat
See values >
4.179
water
heat energy
Joules (J)
0.900
aluminum
+ means energy gained
means energy lost
0.385
copper
т
Grams (g)
iron
0.450
mass
4T
Change in temp
Tr-Ti (°C)
granite
0.790
1. When 3.0 kg of water is cooled from 80.0°C to 10.0°C, how much heat energy is losť?
Answer
Knowns and unknown (?)
Plug values into formula
Cp= 4,18
q=
4T=
2. How much heat is needed to raise a 0.30 kg piece of aluminum from 30.°C to 150°C?
Answer
Knowns and unknown (?)
Plug values into formula
q=
Cp=
AT=
3. Calculate the temperature change when:
a) 10.0 kg of water loses 232 kJ of heat.
Solve for unknown
Answer
Knowns and unknown (?)
Plug values into formula
Cp=
=b
AT=
m=
b) 1.96 kJ of heat are added to 500. g of copper.
Plug values into formula
Solve for unknown
Answer
Knowns and unknown (?)
Cp=
AT=

Transcribed Image Text:*when heated, the temperature of a water sample increased from 15°C to 39°C. It absorbed 4300 joules of
heat. What is the mass of the sample?
3. 3.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu?
0. The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release
of 47 Joules of heat. What is the specific heat of iron?
7. The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 Joules of heat.
What is the mass of the sample?
8. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to
change from 10°C to 29°C?
9. If a 3.1g ring is heated using 10.0 J, its temperature rises 17.9°C. Calculate the specific heat capacity of
the ring.
10. What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its
temperature changes from 25°C to 20°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning