SPOT TEST OBSERVATION MATRIX Reagent K.Cro, KSCN NH,OH DMG HCI H,SO, Yellow Clear Clear precipitate No precipitate precipitate No precipitate No reaction precipitate Clear White White Pb(NO,), Orange Dark red Faint orange Clear Clear Clear Fe(NO,), precipitate No precipitate precipitate No reaction No precipitate No precipitate Clear precipitate No precipitate No reaction Yellow no Clear Pink solution Cream White Ni(NO,), solution precipitate No reaction Reddish White Faintly milky white precipitate Clear White No precipitate precipitate precipitate Clear AGNO brownish precipitate precipitate • Clear light yellow No precipitate No precipitate No reaction No precipitate solution Opaque Clear Clear Clear White Ba(NO,), precipitate Undereach reagentindicate whathappened (if anything) when mixed with the metal ion shown on the left. Write balanced reactions to representwhat happenedineach spottest.
SPOT TEST OBSERVATION MATRIX Reagent K.Cro, KSCN NH,OH DMG HCI H,SO, Yellow Clear Clear precipitate No precipitate precipitate No precipitate No reaction precipitate Clear White White Pb(NO,), Orange Dark red Faint orange Clear Clear Clear Fe(NO,), precipitate No precipitate precipitate No reaction No precipitate No precipitate Clear precipitate No precipitate No reaction Yellow no Clear Pink solution Cream White Ni(NO,), solution precipitate No reaction Reddish White Faintly milky white precipitate Clear White No precipitate precipitate precipitate Clear AGNO brownish precipitate precipitate • Clear light yellow No precipitate No precipitate No reaction No precipitate solution Opaque Clear Clear Clear White Ba(NO,), precipitate Undereach reagentindicate whathappened (if anything) when mixed with the metal ion shown on the left. Write balanced reactions to representwhat happenedineach spottest.
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
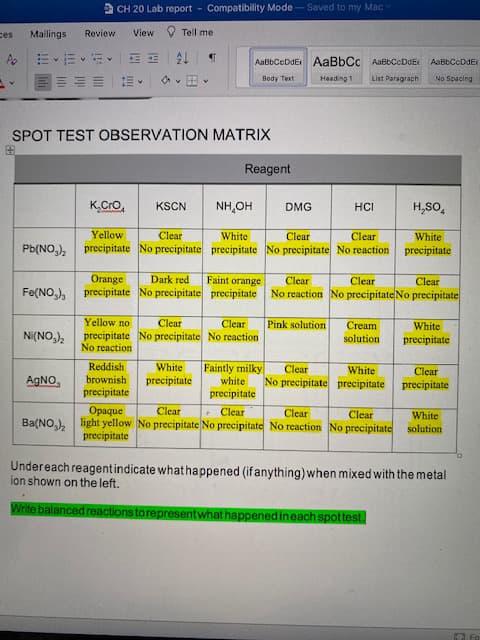
Transcribed Image Text:SPOT TEST OBSERVATION MATRIX
Reagent
K.Cro,
KSCN
NH,OH
DMG
HCI
H,SO,
Yellow
Clear
Clear
precipitate No precipitate precipitate No precipitate No reaction precipitate
Clear
White
White
Pb(NO,),
Orange
Dark red
Faint orange
Clear
Clear
Clear
Fe(NO,),
precipitate No precipitate precipitate No reaction No precipitate No precipitate
Clear
precipitate No precipitate No reaction
Yellow no
Clear
Pink solution
Cream
White
Ni(NO,),
solution
precipitate
No reaction
Reddish
White
Faintly milky
white
precipitate
Clear
White
No precipitate precipitate precipitate
Clear
AGNO
brownish
precipitate
precipitate
• Clear
light yellow No precipitate No precipitate No reaction No precipitate solution
Opaque
Clear
Clear
Clear
White
Ba(NO,),
precipitate
Undereach reagentindicate whathappened (if anything) when mixed with the metal
ion shown on the left.
Write balanced reactions to representwhat happenedineach spottest.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

