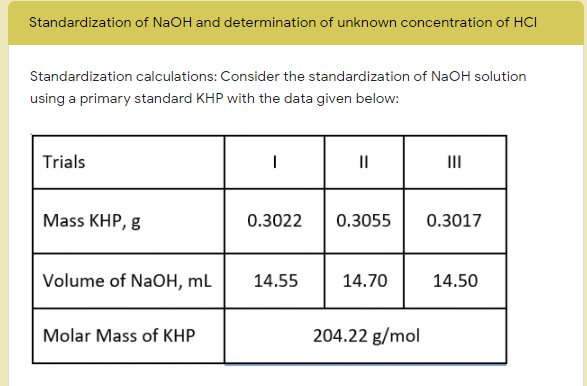
Standardization calculations: Consider the standardization of NaOH solution using a primary standard KHP with the data given below: Trials II II Mass KHP, g 0.3022 0.3055 0.3017 Volume of NaOH, mL 14.55 14.70 14.50 Molar Mass of KHP. 204.22 g/mol
Standardization calculations: Consider the standardization of NaOH solution using a primary standard KHP with the data given below: Trials II II Mass KHP, g 0.3022 0.3055 0.3017 Volume of NaOH, mL 14.55 14.70 14.50 Molar Mass of KHP. 204.22 g/mol
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
What is the trial 1, trial 2 and trial 3 of this
Transcribed Image Text:Standardization of NaOH and determination of unknown concentration of HCI
Standardization calculations: Consider the standardization of NaOH solution
using a primary standard KHP with the data given below:
Trials
II
II
Mass KHP, g
0.3022
0.3055
0.3017
Volume of NaOH, mL
14.55
14.70
14.50
Molar Mass of KHP
204.22 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

