Study Questions 137h Finally, the presence of arsenic is confirmed by adding AGNO3 to the solution of H3ASO4 to precipitate a reddish brown solid Ag,AsO,. The composition of this solid is As, 16.199% and Ag, 69.964%. (a) What are the oxidation numbers of As, S, and N in the reaction of As2S3 with nitric acid? (b) What is the formula of the reddish brown solid Ag,AsO,? Volume of added Volume of added sulfuric acid sulfuric acid 94./ You have a bottle of solid barium hydroxide and some dilute sulfuric acid. You place some of the barium hydroxide in water and slowly add sulfuric acid to the mixture. While adding the sulfuric acid, you measure the conductivity of the mixture. (a) (b) (a) Write the complete, balanced equation for the reac- tion occurring when barium hydroxide and sulfuric acid are mixed. (b) Write the net ionic equation for the barium hydrox- ide and sulfuric acid reaction. Volume of added sulfuric acid Volume of added (c) Which diagram represents the change in conductivity the sulfuric acid barium hydrox- (d) as the acid is added to aqueous (c) ide? Explain briefly Electrical conductivity Electrical conductivity Electrical conductivity Electrical conductivity
Study Questions 137h Finally, the presence of arsenic is confirmed by adding AGNO3 to the solution of H3ASO4 to precipitate a reddish brown solid Ag,AsO,. The composition of this solid is As, 16.199% and Ag, 69.964%. (a) What are the oxidation numbers of As, S, and N in the reaction of As2S3 with nitric acid? (b) What is the formula of the reddish brown solid Ag,AsO,? Volume of added Volume of added sulfuric acid sulfuric acid 94./ You have a bottle of solid barium hydroxide and some dilute sulfuric acid. You place some of the barium hydroxide in water and slowly add sulfuric acid to the mixture. While adding the sulfuric acid, you measure the conductivity of the mixture. (a) (b) (a) Write the complete, balanced equation for the reac- tion occurring when barium hydroxide and sulfuric acid are mixed. (b) Write the net ionic equation for the barium hydrox- ide and sulfuric acid reaction. Volume of added sulfuric acid Volume of added (c) Which diagram represents the change in conductivity the sulfuric acid barium hydrox- (d) as the acid is added to aqueous (c) ide? Explain briefly Electrical conductivity Electrical conductivity Electrical conductivity Electrical conductivity
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 69E: Outline the steps needed to solve the following problem, then do the calculations. Ether, (C2H5)2O,...
Related questions
Question
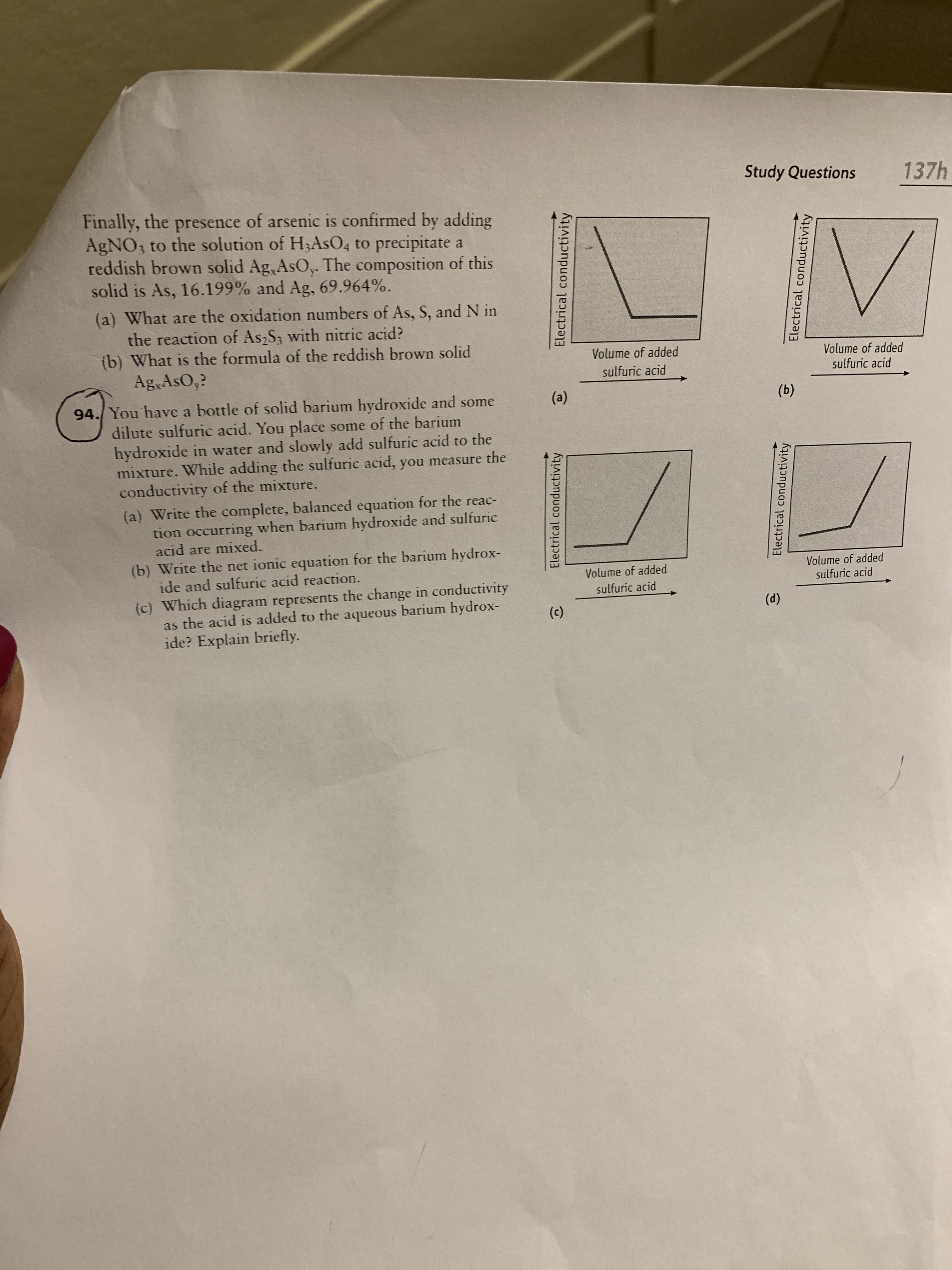
Transcribed Image Text:Study Questions
137h
Finally, the presence of arsenic is confirmed by adding
AGNO3 to the solution of H3ASO4 to precipitate a
reddish brown solid Ag,AsO,. The composition of this
solid is As, 16.199% and Ag, 69.964%.
(a) What are the oxidation numbers of As, S, and N in
the reaction of As2S3 with nitric acid?
(b) What is the formula of the reddish brown solid
Ag,AsO,?
Volume of added
Volume of added
sulfuric acid
sulfuric acid
94./ You have a bottle of solid barium hydroxide and some
dilute sulfuric acid. You place some of the barium
hydroxide in water and slowly add sulfuric acid to the
mixture. While adding the sulfuric acid, you measure the
conductivity of the mixture.
(a)
(b)
(a) Write the complete, balanced equation for the reac-
tion occurring when barium hydroxide and sulfuric
acid are mixed.
(b) Write the net ionic equation for the barium hydrox-
ide and sulfuric acid reaction.
Volume of added
sulfuric acid
Volume of added
(c) Which diagram represents the change in conductivity
the
sulfuric acid
barium hydrox-
(d)
as the acid is added to
aqueous
(c)
ide? Explain briefly
Electrical conductivity
Electrical conductivity
Electrical conductivity
Electrical conductivity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning