Substance Reaction to flame Phase at room temperature Reaction with water Hydrogen Colorless Gas None Explodes Bubbling and reaction is hot. Potassium Burns and is likely to explode Silver Solid Sulfur Yellow Solid None Burns slowly and turns a color Strontium chloride White Crystalline Solid Dissolves Melts Which substance and explanation proves that no chemical change took place? O A Hydrogen because it did not react with water and will explode when exposed to a flame. O B. Potassium because it bubbles in water and will explode when exposed to a flame ondernin-
Substance Reaction to flame Phase at room temperature Reaction with water Hydrogen Colorless Gas None Explodes Bubbling and reaction is hot. Potassium Burns and is likely to explode Silver Solid Sulfur Yellow Solid None Burns slowly and turns a color Strontium chloride White Crystalline Solid Dissolves Melts Which substance and explanation proves that no chemical change took place? O A Hydrogen because it did not react with water and will explode when exposed to a flame. O B. Potassium because it bubbles in water and will explode when exposed to a flame ondernin-
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.88PAE
Related questions
Question
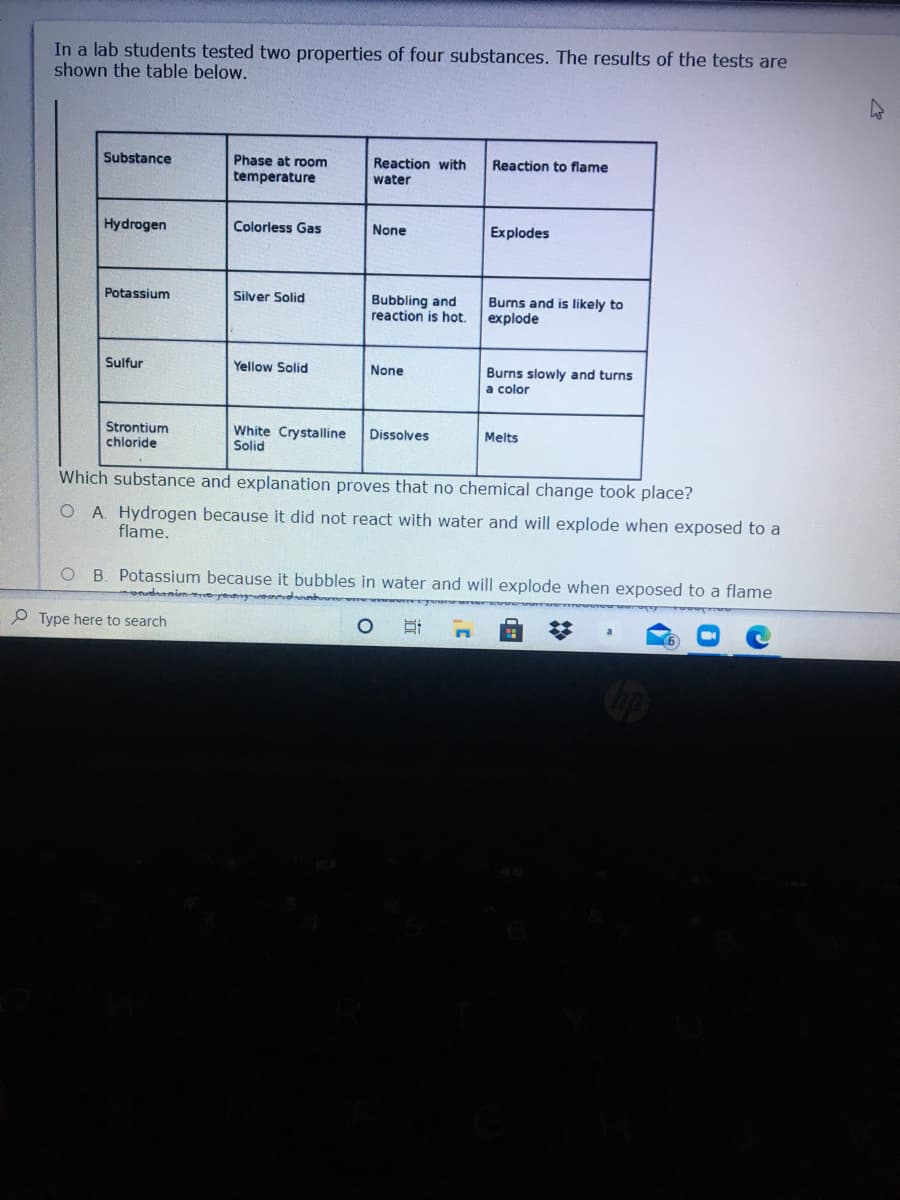
Transcribed Image Text:In a lab students tested two properties of four substances. The results of the tests are
shown the table below.
Substance
Phase at room
Reaction with
Reaction to flame
temperature
water
Hydrogen
Colorless Gas
None
Explodes
Potassium
Silver Solid
Bubbling and
reaction is hot.
Burns and is likely to
explode
Sulfur
Yellow Solid
None
Burns slowly and turns
a color
Strontium
chloride
White Crystalline
Solid
Dissolves
Melts
Which substance and explanation proves that no chemical change took place?
O A. Hydrogen because it did not react with water and will explode when exposed to a
flame.
O B. Potassium because it bubbles in water and will explode when exposed to a flame
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning