Suppose a 250. mL flask is filled with 1.5 mol of CO, 1.8 mol of H,0 and 0.10 mol of H,. This reaction becomes possible: co(g) + H,0(g) - Co,() +H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,. You can leave out the M symbol for molarity. co H20 co, H, initial change equilibrium olo x
Suppose a 250. mL flask is filled with 1.5 mol of CO, 1.8 mol of H,0 and 0.10 mol of H,. This reaction becomes possible: co(g) + H,0(g) - Co,() +H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,. You can leave out the M symbol for molarity. co H20 co, H, initial change equilibrium olo x
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 22QAP: An experiment calls for a 0.4500 M solution of aluminum carbonate. The storeroom has only 32.00 g of...
Related questions
Question
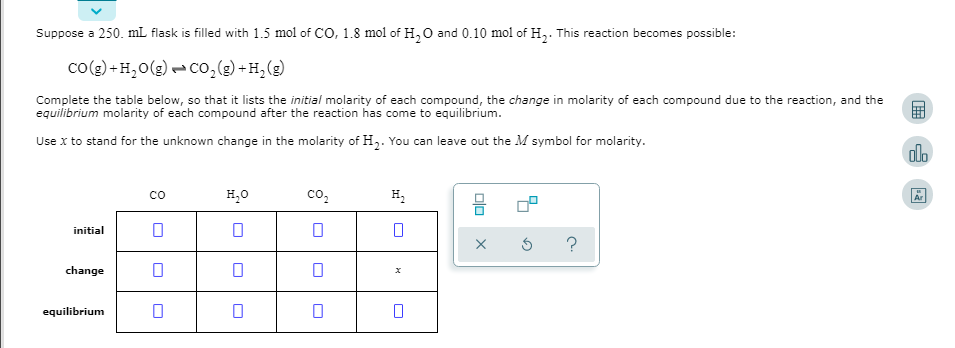
Transcribed Image Text:Suppose a 250. mL flask is filled with 1.5 mol of CO, 1.8 mol of H,0 and 0.10 mol of H,. This reaction becomes possible:
co(g) + H,0(g) - Co,() +H,(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of H,. You can leave out the M symbol for molarity.
co
H20
co,
H,
initial
change
equilibrium
olo x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning