Suppose a powerful battery is connected between a pair of inert graphite electrodes dipped into a pot of molten sodium chloride (NaCl) (see sketch at right). The battery makes a current of electrons flow in the directions shown by the arrows in the sketch. Note: Group 1A and Group 2A metals all melt at temperatures lower than their salts. A В OA Which electrode is the cathode? O-0 O B Write a balanced equation for the half-reaction that takes place at electrode A. Write a balanced equation for the overall reaction.
Suppose a powerful battery is connected between a pair of inert graphite electrodes dipped into a pot of molten sodium chloride (NaCl) (see sketch at right). The battery makes a current of electrons flow in the directions shown by the arrows in the sketch. Note: Group 1A and Group 2A metals all melt at temperatures lower than their salts. A В OA Which electrode is the cathode? O-0 O B Write a balanced equation for the half-reaction that takes place at electrode A. Write a balanced equation for the overall reaction.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter32: Voltaic Cell Measurements
Section: Chapter Questions
Problem 2ASA
Related questions
Question
100%
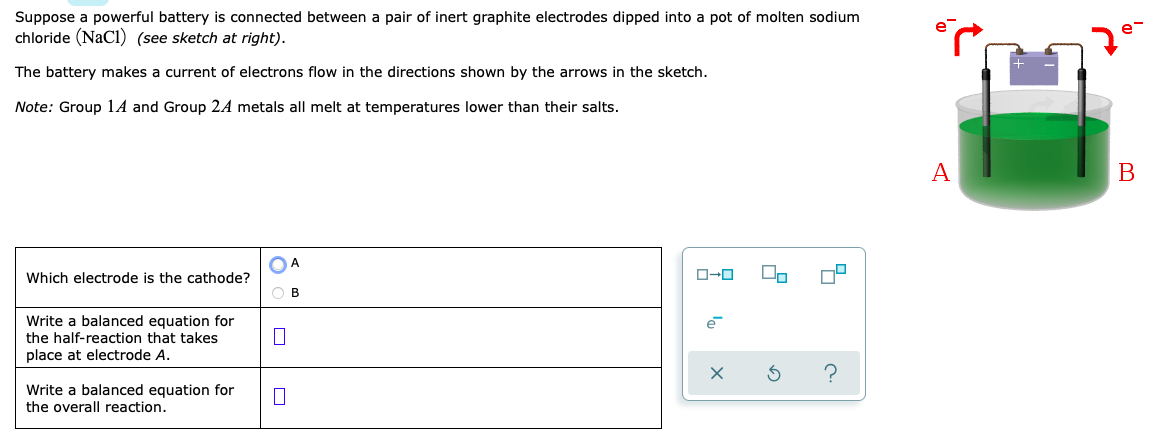
Transcribed Image Text:Suppose a powerful battery is connected between a pair of inert graphite electrodes dipped into a pot of molten sodium
chloride (NaCI) (see sketch at right).
The battery makes a current of electrons flow in the directions shown by the arrows in the sketch.
Note: Group 1A and Group 2A metals all melt at temperatures lower than their salts.
A
A
Which electrode is the cathode?
O B
Write a balanced equation for
the half-reaction that takes
place at electrode A.
e
Write a balanced equation for
the overall reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
