Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl,(aq) + 2 AgNO3(aq) 2 AgCl(s) + Cd(NO3),(aq) The chemist adds 65.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 5.4 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. mg L
Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl,(aq) + 2 AgNO3(aq) 2 AgCl(s) + Cd(NO3),(aq) The chemist adds 65.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 5.4 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. mg L
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 53A
Related questions
Question
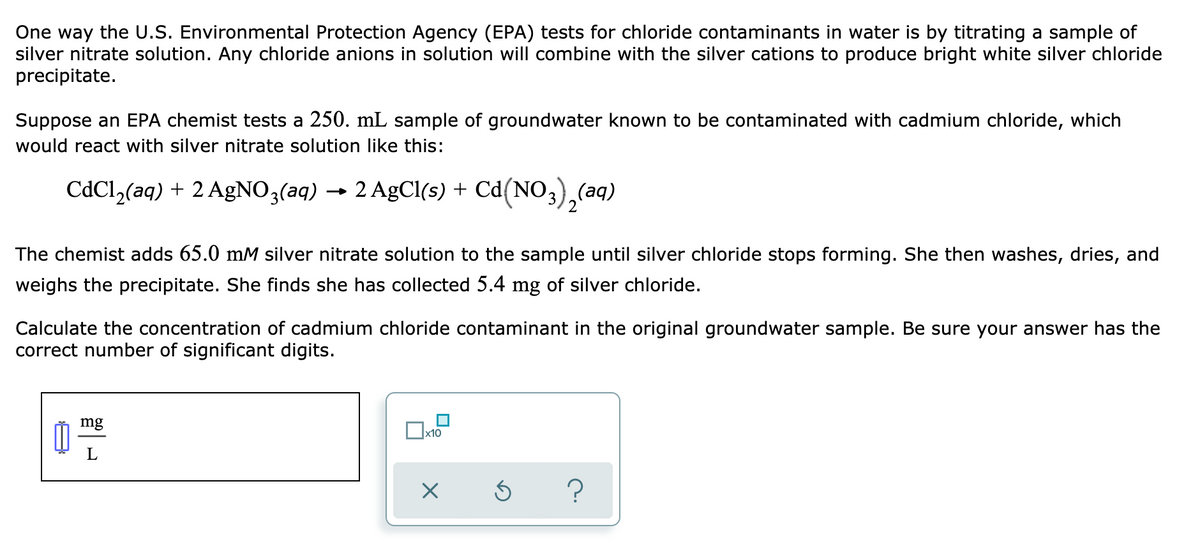
Transcribed Image Text:One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of
silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride
precipitate.
Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with cadmium chloride, which
would react with silver nitrate solution like this:
CdCl,(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Cd(NO3),(aq)
The chemist adds 65.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and
weighs the precipitate. She finds she has collected 5.4 mg of silver chloride.
Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the
correct number of significant digits.
mg
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax