System Stopcock closed Vacum
Q: In the equation z = ct + d, z is measured in meters and t is measured in seconds. What are the dimen...
A: Dimensional Analysis: Base quantities are physical quantities that cannot be expressed using any oth...
Q: Dry air is a pretty decent insulator; it has a very high resistivity of 3 X 10 m. Consider a capacit...
A: Given, ρ=3×1013 Ω.mside of square, a=15 cm=0.15 md=0.8 mm= 8×10-4 mv=320 v c=ε0 Ad=8.85×10-12×0.15×...
Q: Electric field A L %3D 1 2 H foietionles the Electrie charge es pulled by with mas m a force F. Bete...
A: Solution: let the charge with mass m is initially at rest at point A. A constant force F is applied ...
Q: 3 : A body of moment of inertia of 3 kg-m² rotating with an angular velocity of 2 rad/s the same kin...
A: In this case, the moment of inertia and the angular velocity of a body is given. On the other hand, ...
Q: Get the period and frequency of a simple harmonic vibration from: 1. Spring mass oscillation a) with...
A: The simple harmonic motion is described as a periodic motion where the acceleration is proportional ...
Q: A = 5 units, E B = 10 units, N = 12 units, 30° N of W D = 5 units, SE
A: Given, A→=5 units , EB→=10 units. NC→=12 units, 30° N of WD→=5 units, SE
Q: The cantilever is having one side of it as fixed while the other one is Select one: a. pin support b...
A: A Cantilever is a structural beam or bridge that is supported at one end only and the other end rem...
Q: (Q) Consider a system of three noninteracting particles that are confined to move in a one-dimension...
A: energy of the particle inside energy level n is given by E=n2π2h22mL2 where m is the mass of the par...
Q: 25. Write short notes on the follówing: (i) Einstein's velocity addition theorem (ii) Relativistic t...
A:
Q: Two small balls, each of mass 5.4 g, are attached to silk threads 46 cm long, which are in turn tied...
A: Given m=5.4g=5.4x10-3kg, L=46cm=0.46m θ=5.0⁰ The two masses have same magnitude charge Q.
Q: 9: A stretched sonoIMeier wire is in unison with a tuning fork. When the length is increased by 2% t...
A:
Q: What is Minkowski space ? Give the concept of fow 1atn anerov momentum fr
A: It is represented as,
Q: An Argo probe is drifting in a Pacific Ocean current with a velocity of 60 cm/s [W] relative to the ...
A: Given, Velocity of water Vw = 60 cm/s [w] The velocity of the probe with respect to water Vp = 40 cm...
Q: **** v u war. o g. 23. Using Maxwell's equations show that electric and magnetic fields propagate in...
A: Solution: The Maxwell equation's written as
Q: Determine the y component of the force F3 shown below in the figure. (in kN, 2 decimal places, inclu...
A: A vector is a quantity that is defined by both a magnitude and a direction. Some examples of vector ...
Q: A car drives past a police radar. Calculate the car's speed if the police measure an angular velocit...
A: Given that,Angular velocity, θ˙=-0.246 rad/secr˙=7.81 m/sRadius, r=57.7 mWe know that,The velocity o...
Q: 2. (a) An object undergoing SHM traces a parabola taking 0.25 s to travel from one point of minimum ...
A: Solution 2: a). In Simple harmonic motion, the particle changes its velocity and there is a transiti...
Q: 74 : A train rounds a curve of radius 150 m at a speed of 20 m/s. Calculate the angle rails. Also fi...
A: In this question we have to find the angle of baking θ and the elevation (h) of the outer rail over ...
Q: Given the mass of the cart m,=50 kg and the mass of the wheel m=25 kg and its radius r=0.25 m. The %...
A: Given data, Mass of the cart = mc = 50 kg Mass of the wheel = mw = 25 kg Radius of the wheel = r = 0...
Q: A circuit has a capacitor C, a resistor R, an inductor L, and a key, all connected in series. When t...
A: Given: The LCR circuit.
Q: Qamma utr aviolet intrared Xays radar FM TV shortwave AM Tays Tays Fays 10 10 10 10 10 10 10 Wavelen...
A: According to the electromagnetic spectrum Visible light falls in the range of 400 nm to 700 nm range...
Q: The symbol for charge is Q. True False Question 2 Polarization is the separation of charge in an obj...
A:
Q: 4. B=30T |= 20 A 37° | (Lab= Lbc= Lcd=Lde= 2 m) A bended wire, which is carring total current of 20A...
A: Given: Four line segment carrying current of 20 A Magneetic field is 30T and Length of each segment ...
Q: Cathode ray tubes (CRTs) used in old-style televisions have been replaced by modern LCD and LED scre...
A: Solution: The given information is: The accelerating potential between the plates is V = 30 kV. The...
Q: A. Calculate the wavelength of light in nanometer with a frequency of 7.83x10-19J per photon. B. Ref...
A: In this case, the energy per photon is given. For this energy, we have to calculate the correspondin...
Q: What does the appearance look like when the director is at 30° to the polariser direction?
A: Malus Law: It states for a plane-polarized light when it is passed through an analyzer its intensity...
Q: Q. 26 : The equation of displacement of particle performing SHM is maximum velocity is x = 0.25 sin ...
A: Given Data : frequency (n) = 0.002 Hz (n) = 21000 Hz We...
Q: A 5.0 kg object is pulled along a horizontal surface at a constant speed by a 15 N force acting 20 d...
A: Given Mass (m) = 5.0 kg Force (F) = 15 N Angle θ = 20° Displace...
Q: 89 : The length of simple pendulum is increased by 44%. Determine the % increase in time period.
A: Description : In this question we have to determine the percent increase in time period for the simp...
Q: a.) Who is to furnish the outlet boxes required at each telephone outlet? b.) Who is to furnish the...
A: Outlet Box: A terminal box for electric wiring or fittings where the wires terminate for connection ...
Q: Solve the given equation by the frobenius method and show that for some values of the constant ^ we ...
A: A differential equation is an equation that the derivatives one (or more) variable to another. The d...
Q: 14. A body moving with velocity v has a mass m. Show that mo m = where mo is the rest mass of the bo...
A:
Q: A wire 4 m long and 0.2 mm in diameter is stretched by a force of 8 kg. Wt. If the wire elongates by...
A: Description : In this case we have to find the energy stored in the wire by having length of wire 4 ...
Q: zp cos o a, C/m" Calculate the total charge enclosed by the cylinder of radius 1 m with -2 <z <2 m. ...
A: Given, D=zp cos 2ϕazC/m2radius =1m from Gauss Law we have, Q=∫D.ds=ϕ1+ϕ2+ϕ3where, ϕ1,ϕ2,ϕ3 are the f...
Q: F1-500 kips F2 69 1 0.8 F3=250 kips Three concurrent forces are acting as shown. If F2-268.0 and =29...
A:
Q: aw³ sin(wt + 65°) . Determine the Suppose, the instantaneous displacement of a SHM is x = total ener...
A: Given: x = aω3sin(ωt+65o) We have to find total energy of SHM
Q: Please help with the solution. Give an example of when you’d use a combined plane change vs a si...
A: Concept used: The change in inclination of orbit of orbiting body is called orbital inclination chan...
Q: A volume of 58.0 L of hydrogen is heated from 33°C to 68°C. If its original density is 4.85 kg/m3 an...
A: Given that,The initial volume : V1 = 58×10-3The initial temperature : T1 = 33°CThe final temperature...
Q: One kilogram of water at 0 C is heated to 100 C. Compute its change in entropy. Assume that the spec...
A: Given that,Mass of water : m = 1 (kg)Heat : C = 4190 (J/kgK)Temperature: T2 = 100°CTemperature: T1 ...
Q: Define thin film and explain the phenomenon of interference in thin film due to reflected rays.
A: (a) Thin film. (b) The phenomenon of interference in thin film due to reflected rays.
Q: The parallel axis theorem uses the of the distance
A: Required : The parallel axis theorem uses ----- of the distance.
Q: Example 4: The initial voltages on capacitors C and C2 in the circuit shown have been established by...
A: Given, Capacitance, C1=5μF=5×10-6FC2=20μF=20×10-6F Since both are in series, then equivalent capacit...
Q: 14. Derive the Lorentz transformations for electromagnetic potential four vector and current density...
A: Solution: Maxwell's field equation is given as Where The second equation can be written as
Q: A particle of mass m = 1.0 mg and charge 2.0 µC is placed in a region of space that has a constant e...
A: Given that:m=1 mg=10-6 kgQ=2 μC= 2×10-6 CE=2000 N/C
Q: A wire having young's modulus 1.2 x 1011 N/m2 is subjected to the stress of 2.4 x 107 N/m2. If the l...
A: Given data : Young's modulus (Y)=1.2×1011 N/m2 Tensile stress Mgπr2=2.4×107 N/m2...
Q: A circular disc of mass M and radius R is rotating about its axis with angular speed ω1 . If another...
A:
Q: (a) Determine the electric field strength at a point 1.00 cm to the left of the middle charge shown ...
A: Given, changes are, QA=6μC=6×10-6CQB=1.5μC=1.5×10-6CQC=-2μC=-2×10-6C D is a point placed at 1 cm fr...
Q: 1) Consider the model from a different group, similar to but not the same as Group 2's model: SSNN S...
A: The question is basically asking that in given observations which is difficult to explain throug...
Q: Suppose 3.1 mol of an ideal gas of volume V1 = 4.1 m^3 at T1 = 305 K, it is allowed to expand isothe...
A: Given that moles of the gas is n=3.1 Initial volume is V1=4.1 m3 Final volume is V2=8.00 m3 final...
Q: Q8. The points A, B and C in the circuit below can be individually switched to connect either to +V ...
A: In the logic Function GND =0+V=1
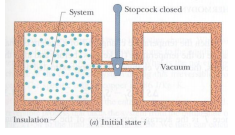
a) Suppose 1.0 mole of nitrogen is confined to the left side of the container in the figure below. The valve is opened and the volume of the gas doubles. What is the entropy change of the gas in this irreversible process? Treat gas as ideal.
b) After having calculated what was asked in letter a, answer what is the variation of entropy if the gas had expanded, doubling the volume, through a free and adiabatic expansion? Justify your answer, explain the differences and similarities between the cases and what is linked to the entropy variation in this case.
Step by step
Solved in 3 steps
