Table 10.2 Samples Placed in Water (data) Sample Dialysis tubing containing sugar solution ass Before Soakin lon 5.083 Prune Mass After Soaki 5.480 12.652 11.278 Table 10.3 (report) hange in Sample as urroundin irectiono onc. of Solution (inside sample Sample lativ en Dialysis tubing + water Dialysis tubing + sugar solution Prune Sugar solution Pure water Sugar solution Pure water Prune
Table 10.2 Samples Placed in Water (data) Sample Dialysis tubing containing sugar solution ass Before Soakin lon 5.083 Prune Mass After Soaki 5.480 12.652 11.278 Table 10.3 (report) hange in Sample as urroundin irectiono onc. of Solution (inside sample Sample lativ en Dialysis tubing + water Dialysis tubing + sugar solution Prune Sugar solution Pure water Sugar solution Pure water Prune
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.44E: Are the following processes examples of homogeneous or heterogeneous catalysis? a Hydrolysis of...
Related questions
Question
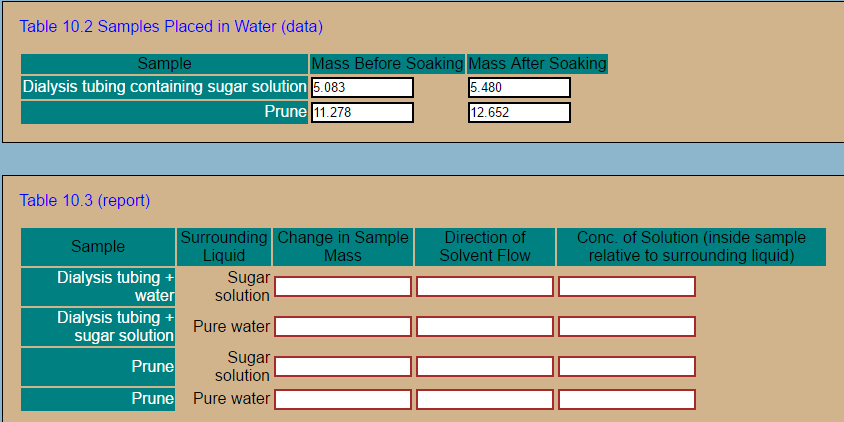
Transcribed Image Text:Table 10.2 Samples Placed in Water (data)
Sample
Dialysis tubing containing sugar solution
ass Before Soakin
lon 5.083
Prune
Mass After Soaki
5.480
12.652
11.278
Table 10.3 (report)
hange in Sample
as
urroundin
irectiono
onc. of Solution (inside sample
Sample
lativ
en
Dialysis tubing +
water
Dialysis tubing +
sugar solution
Prune
Sugar
solution
Pure water
Sugar
solution
Pure water
Prune
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,