TABLE 10.3 Van der Waals Constants for Gas Molecules Substance a (L²-atm/mol?) b (L/mol) Не 0.0341 0.02370 Ne 0.211 0.0171 Ar 1.34 0.0322 Kr 2.32 0.0398 Xe 4.19 0.0510 На 0.244 0.0266 N2 1.39 0.0391 O2 1.36 0.0318 F2 1.06 0.0290 Cl, 6.49 0.0562 Н-о 5.46 0.0305 NH3 4.17 0.0371 CH4 2.25 0.0428 CO2 3.59 0.0427 CCI4 20.4 0.1383
TABLE 10.3 Van der Waals Constants for Gas Molecules Substance a (L²-atm/mol?) b (L/mol) Не 0.0341 0.02370 Ne 0.211 0.0171 Ar 1.34 0.0322 Kr 2.32 0.0398 Xe 4.19 0.0510 На 0.244 0.0266 N2 1.39 0.0391 O2 1.36 0.0318 F2 1.06 0.0290 Cl, 6.49 0.0562 Н-о 5.46 0.0305 NH3 4.17 0.0371 CH4 2.25 0.0428 CO2 3.59 0.0427 CCI4 20.4 0.1383
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 70QRT
Related questions
Question
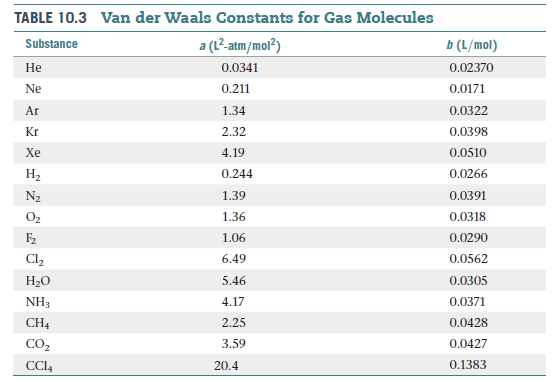
Table 10.3 shows that the van der Waals b parameter has
units of L/mol. This means that we can calculate the sizes
of atoms or molecules from the b parameter. Is the van der Waals radius we
calculate from the b parameter of Table 10.3 more closely
associated with the bonding or nonbonding atomic radius
discussed there? Explain.
Transcribed Image Text:TABLE 10.3 Van der Waals Constants for Gas Molecules
Substance
a (L²-atm/mol?)
b (L/mol)
Не
0.0341
0.02370
Ne
0.211
0.0171
Ar
1.34
0.0322
Kr
2.32
0.0398
Xe
4.19
0.0510
На
0.244
0.0266
N2
1.39
0.0391
O2
1.36
0.0318
F2
1.06
0.0290
Cl,
6.49
0.0562
Н-о
5.46
0.0305
NH3
4.17
0.0371
CH4
2.25
0.0428
CO2
3.59
0.0427
CCI4
20.4
0.1383
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning