Table 5E.1 Successive acidity constants of polyprotic acids at 298.15 K Acid pKa1 K2 pK2 pKa3 Carbonic acid, H,CO3 4.3 x 10-7 6.37 5.6 x 10-11 10.25 1.3 x 10 Hydrosulfuric acid, H,S Ethanedioic acid (oxalic acid), (COOH), 6.88 7.1 x 10-15 14.15 5.9 x 10-2 1.23 6.5 x 10-5 4.19 Phosphoric acid, H3PO4 7.6 x 10-3 2.12 6.2 x 10-8 7.21 2.1 x 10-13 12.67 Phosphorous acid, H,PO3 Sulfuric acid, H,SO4 1.0 x 10-2 2.00 2.6 x 10-7 6.59 Strong 1.2 x 10-2 1.92 Sulfurous acid, H2SO3 1.5 x 10-2 1.81 1.2 x 10-7 6.91 Tartaric acid, C,H,O2(COOH), 6.0 x 10-4 3.22 1.5 x 10-5 4.82
Table 5E.1 Successive acidity constants of polyprotic acids at 298.15 K Acid pKa1 K2 pK2 pKa3 Carbonic acid, H,CO3 4.3 x 10-7 6.37 5.6 x 10-11 10.25 1.3 x 10 Hydrosulfuric acid, H,S Ethanedioic acid (oxalic acid), (COOH), 6.88 7.1 x 10-15 14.15 5.9 x 10-2 1.23 6.5 x 10-5 4.19 Phosphoric acid, H3PO4 7.6 x 10-3 2.12 6.2 x 10-8 7.21 2.1 x 10-13 12.67 Phosphorous acid, H,PO3 Sulfuric acid, H,SO4 1.0 x 10-2 2.00 2.6 x 10-7 6.59 Strong 1.2 x 10-2 1.92 Sulfurous acid, H2SO3 1.5 x 10-2 1.81 1.2 x 10-7 6.91 Tartaric acid, C,H,O2(COOH), 6.0 x 10-4 3.22 1.5 x 10-5 4.82
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.8QAP
Related questions
Question
Calculate the pH of the following acid solutions at 25 °C: (a) 1.0 x 10-4 M H3BO3(aq) (boric acid acts as a monoprotic acid), (b) 0.015 M H3PO4 (aq), (c) 0.10 M H2SO3(aq). Values for the successive acidity constants for each of these acids are given in the below table.
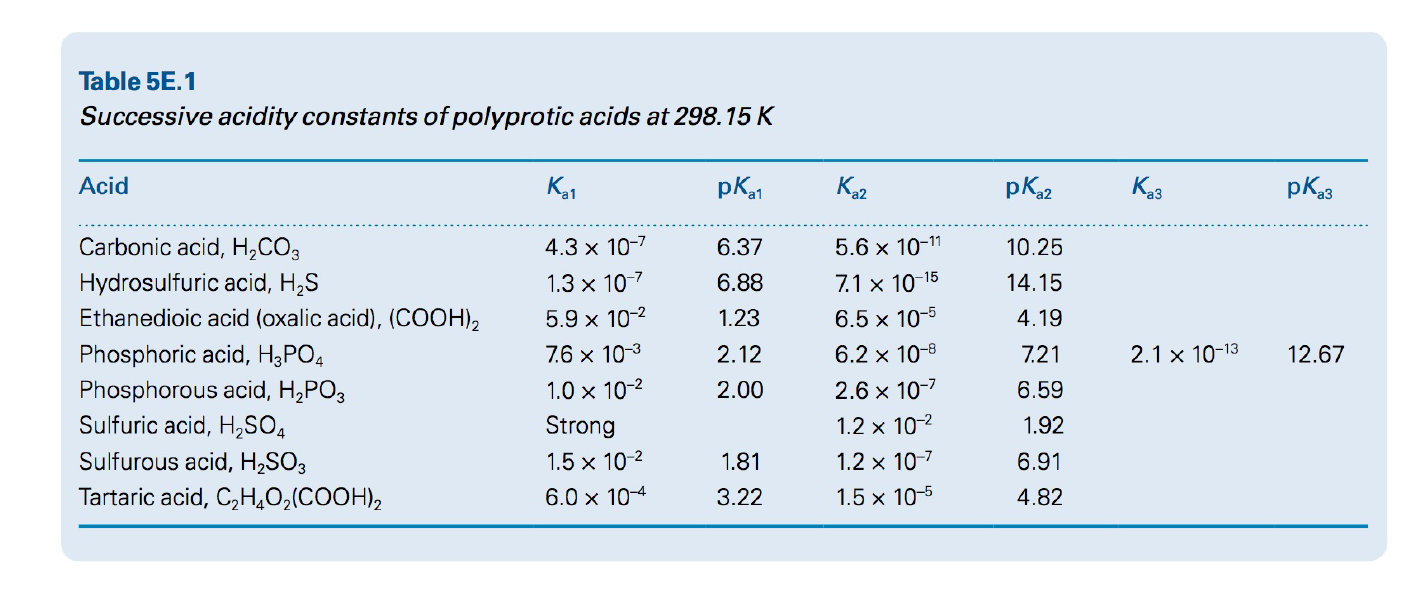
Transcribed Image Text:Table 5E.1
Successive acidity constants of polyprotic acids at 298.15 K
Acid
pKa1
K2
pK2
pKa3
Carbonic acid, H,CO3
4.3 x 10-7
6.37
5.6 x 10-11
10.25
1.3 x 10
Hydrosulfuric acid, H,S
Ethanedioic acid (oxalic acid), (COOH),
6.88
7.1 x 10-15
14.15
5.9 x 10-2
1.23
6.5 x 10-5
4.19
Phosphoric acid, H3PO4
7.6 x 10-3
2.12
6.2 x 10-8
7.21
2.1 x 10-13
12.67
Phosphorous acid, H,PO3
Sulfuric acid, H,SO4
1.0 x 10-2
2.00
2.6 x 10-7
6.59
Strong
1.2 x 10-2
1.92
Sulfurous acid, H2SO3
1.5 x 10-2
1.81
1.2 x 10-7
6.91
Tartaric acid, C,H,O2(COOH),
6.0 x 10-4
3.22
1.5 x 10-5
4.82
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

