TABLE 8.1 Successive Values of lonization Energies for the Elements Sodium through Argon (kJ/mol) Element IE1 IE2 IE3 IE4 IES IES IE7 Na 496 4560 Mg 738 1450 7730 Core electrons Al 578 1820 2750 11,600 Si 786 1580 3230 4360 16,100 P 1012 1900 2910 4960 6270 22,200 S 1000 2250 3360 4560 7010 8500 27,100 CI 1251 2300 3820 5160 6540 9460 11,000 Ar 1521 2670 3930 5770 7240 8780 12,000 Copyright © 2008 Pearson Prentice Hall, Inc. Element Na Mg Al Si P CI Ar Electronic configuration # core e- # valence e- Huge jump is at which IE (eg. second IE)?
TABLE 8.1 Successive Values of lonization Energies for the Elements Sodium through Argon (kJ/mol) Element IE1 IE2 IE3 IE4 IES IES IE7 Na 496 4560 Mg 738 1450 7730 Core electrons Al 578 1820 2750 11,600 Si 786 1580 3230 4360 16,100 P 1012 1900 2910 4960 6270 22,200 S 1000 2250 3360 4560 7010 8500 27,100 CI 1251 2300 3820 5160 6540 9460 11,000 Ar 1521 2670 3930 5770 7240 8780 12,000 Copyright © 2008 Pearson Prentice Hall, Inc. Element Na Mg Al Si P CI Ar Electronic configuration # core e- # valence e- Huge jump is at which IE (eg. second IE)?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter9: Ionic And Covalent Bonding
Section: Chapter Questions
Problem 9.3QP
Related questions
Question
Please help answer this table
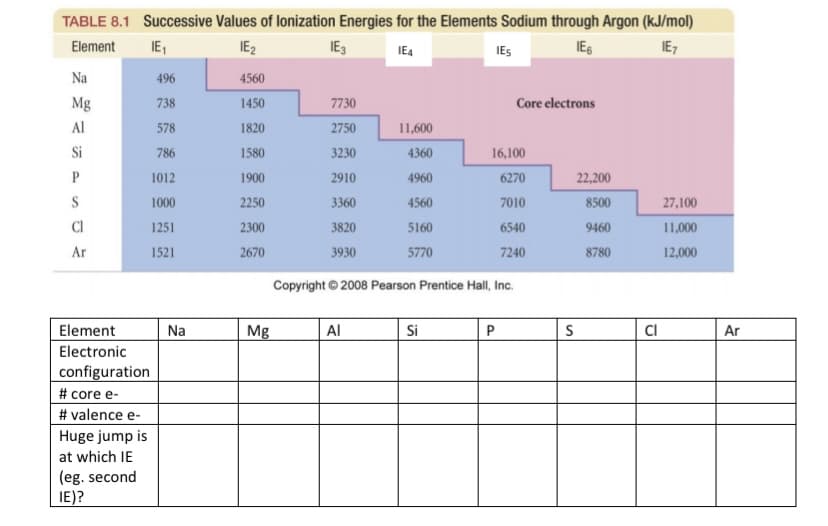
Transcribed Image Text:TABLE 8.1 Successive Values of lonization Energies for the Elements Sodium through Argon (kJ/mol)
IE1
IE2
IE7
Element
IE4
IES
IES
Na
496
4560
Mg
738
1450
7730
Core electrons
Al
578
1820
2750
11,600
Si
786
1580
3230
4360
16,100
P
1012
1900
2910
4960
6270
22,200
S
1000
2250
3360
4560
7010
8500
27,100
CI
1251
2300
3820
5160
6540
9460
11,000
Ar
1521
2670
3930
5770
7240
8780
12,000
Copyright © 2008 Pearson Prentice Hall, Inc.
Element
Na
Mg
Al
Si
P
CI
Ar
Electronic
configuration
# core e-
# valence e-
Huge jump is
at which IE
(eg. second
IE)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER