Taking into account that dG = Vdp –SdT A) write the Maxwell relation between changes in volume and entropy What is true about temperature? A) V = (ôp/ôS)T B) V = (ôG/ôp)v C) V = (ôG/ôT), D) V = (ôS/ôT),
Q: The change in the molar volume accompanying fusion of solid CO2 is -1.6 cm3 mol-1. Determine the…
A:
Q: Estimate the change in the Gibbs energy and molar Gibbs energy of 100 cm3 of water when the pressure…
A:
Q: Given that the Gibbs energy is a function of temperature and pressure G(T, P), estimate the change…
A: Gibbs free energy is a thermodynamic parameter which helps us in finding the maximum work done by a…
Q: The standard state of a substance used to be defined as 1 atm at the specified temperature. By how…
A: Given data:
Q: 5. Calculate the AS when one mole of water is heated from 263 to 283 K given the molar capacities in…
A: Entropies designate randomness and the entropy change arises whenever systems absorbs/release heat.…
Q: Calculate (a) the (molar) Gibbs energy of mixing, (b) the(molar) entropy of mixing when the two…
A:
Q: A sample of water vapour at 200 °c is compressed isothermally from 350 cm3 to 120 cm3. What is the…
A: Change in molar Gibbs energy in a sample of water vapor at 2000C is compressed isothermally from 350…
Q: 5. Under what conditions (a) is dA < 0 the appropriate criterion for spontaneity? (b) is dG <0 the…
A:
Q: V. P. Kolesov et al. (J. Chem Thermodynamics 28 (1996) 1121) reported the standard enthalpy of…
A: The combustion reaction for C60 is given as: C60(s)+60O2(g)→60CO2(g) (1)
Q: Describe how to find the relationshipbetween the state properties (P, V, T ) of agas and its molar…
A: The expression that will be used to describe/find the relationship between the state properties, P,V…
Q: Given a thermodynamic function h=h(s,p) show how to evaluate T, V , U , A or F and G
A: Given function, h=h(S,P)By ditferenciating we get,dh=∂h∂SPds+∂h∂pSdp — (I)From Gibbs realtion, dh…
Q: Given N2 (g) + 3 H2 (g) 2 2 NH3 (g) AG° = -32.90 kJ At 25.0°C, calculate the change in Gibbs energy…
A: The given reaction is,
Q: Estimate the normal boi ling point of benzene given that its vapour pressure is 20 kPa at 35 °C and…
A: Thermodynamic is the branch of chemistry that mainly deals with the heat transfer between system and…
Q: For a certain constant-pressure process, the change in Gibbs energy was found to fit the expression:…
A: Since for any process, the relationship between G, H and S is given by G = H - TS The expression…
Q: 5.Derive Entropy of mixing for an ideal solution derive ASmix= -k(N,LnX,+N„LnXs) using i. Classical…
A: Entropy of mixing, in thermodynamics, is the increase in the total entropy when several initially…
Q: Predict the output of energy as heat from the combustion of 1.0 dm3 of octane at 298 K and 1 bar.…
A:
Q: 2. Assume that some single-component material has the properties of its change in enthalpy and…
A: 2. Given that : At 1.0 atm, Change in enthalpy (∆H) = 52 kJ/mol CHange in entropy (∆S) = 0.16…
Q: At 0 °C liquid water and ice are in equilibrium. What is the effect on the difference in molar Gibbs…
A: Density of ice is 0.9150 g/cm3 Density of water is 0.9999 g/cm3 Gm(pf) and Gm(pi) are related as:
Q: A sample of water vapour at 200 °C is compressed isothermally from 250 cm³ to 114 cm³. What is the…
A:
Q: a) Find the entropy change associated with melting pure ice at T=273.15K, P=20 bar. b) What is the…
A: Melting of pure ice can be represented as: H2O(s)→H2O(l)…
Q: By equating the cross derivatives, which of the following Maxwell relations can you derive from the…
A:
Q: 78.1 g of C6 H,OH(s) at 300. K are transformed to C6 H,OH(1) at 320. K with an increase in entropy…
A: The enthalpy of fusion is the energy required for changing a substance from its solid state to its…
Q: Estimate the change in the Gibbs energy of 100cm3 of water when the pressure acting on it is…
A: Volume of water = 100cm^3 Initial pressure = 100kPa Final Pressure = 500kPa Mass density of water =…
Q: Without doing a calculation, decide whether the presence of (a) attractive, (b) repulsive…
A: Introduction: Gibbs free energy: In a thermodynamic system, the maximum amount of work done at…
Q: 1) Calculate the effect (change) on the chemical potential for liquid carbon dioxide, which had an…
A: Since you have posted a question with multiple questions, we will solve first question for you. To…
Q: Perfect gas AS = nR ln(V/V,) Entropy of isothermal expansion
A: To prove this equation , we would use law of thermodynamics and perfect gas law.
Q: The change in the molar volume accompanying fusion of solid CO2 is −1.6 cm3 mol−1. Determine the…
A: At constant temperature, the relationship between change in molar Gibbs energy, molar volume, and…
Q: Calculate the change of entropy in the system: Delta S of the surrounding=251 kJ/mol, Gibbs free…
A: It is considered that the given system and its surrounding are in thermal equilibrium, then…
Q: What does the superscript ° indicate when associated with a thermodynamicquantity, as in ΔH°, ΔS°,…
A: The superscipt ˚ in thermodynamics indicates that the given thermodynamic quantity is in standard…
Q: The electrical component of the Gibbs energy (i.e. the reversible electrical work) required to move…
A: Since, ∆Gelec have a change of -3.42×105 J in the temperature range from T = 273K to T= 373K. Using…
Q: Data for ammonia: Molar mass = 17.03 g mol1 AvapH = 23.35 kJ mol-1 at the standard boiling point, T,…
A: A solution consists of solute and solvent. A solute is defined as the component that is present in a…
Q: Predict the output of energy as heat from the combustion of 1.0 dm3 of octane at 298 K and 1 bar.…
A: The number of moles of octane in the system is calculated as shown below. m=density×volume1 dm3=1000…
Q: A sample of water vapour at 200 °C is compressed isothermally from 288 cm³ to 67 cm³. What is the…
A:
Q: 9) Estimate the change in the Gibbs energy of 100 cm³ of water when the pressure acting on it is…
A: Gibbs free energy is also known as free energy: It is defined as the measure of the maximum…
Q: The standard Gibbs energy of formation of rhombic sulfur is zero, and that of monoclinic sulfur is…
A: ∆G˚ for rhombic ‘S’ = 0 ∆G˚ for monoclinic ‘S’ = +0.33 kJ/mol Standard molar entropy, Sm for rhombic…
Q: A sample of water vapour at 200 C is compressed isothermally from 302c * m ^ 3 to 58c * m ^ 3 What…
A: We can solve this problem by using following equation ∆G= RTln(V1/V2) Where ∆G= change in molar…
Q: The coefficient of fugacity of percent gas at 290 K and 2.1 MPa is 0.68. Calculate the molar Gibbs…
A:
Q: (a) The standard molar entropy of ethene is 220 J K-' mol·' (with a standard state of 1 bar).…
A: standard molar entropy of ethene (So) = 220 J/K mol moles of ethene (n) = 1.75 mol pressure (P) = 1…
Q: What is the value of C_p for Cl_2, vapor using the full statistical theory at 298 K. v_0 = 559.7…
A:
Q: Q5:- The value of entropy (in meV/K units) when the general mode weight of the assembly is 1600,…
A:
Q: What does the superscript ° indicate when associated with a thermodynamicquantity, as in ∆H °, ∆S°,…
A: The superscipt ˚ in thermodynamics indicates that the given thermodynamic quantity is in standard…
Q: 1.Calculate the effect (change) on the chemical potential for liquid carbon dioxide, which had an…
A: Chemical potential determine the stability of the substances and is directly proportional to the…
Q: The change of molar volume accompanying fusion of a solid organic compound is 0.55 cm³mol-1.…
A: Given, change in molar volume = 0.55 cm3mol increase in pressure = 7 to 4200 bar
Q: This question deals with the vapourization of isopropyl alcohol: CH;CH(OH)CH3(1) =CH3CH(OH)CH3(g)…
A: Given reaction is : CH3CH(OH)CH3 (l) <-------------------> CH3CH(OH)CH3 (g) Given change in…
Q: 1) Calculate the effect (change) on the chemical potential for liquid carbon dioxide, which had an…
A: In most of the conditions when we raise the pressure, it will definitely lead to a greater increase…
Q: Suppose now that argon is added to the mixture in the previous exercise to bring the composition…
A: Gibbs free enrgy of mixture is given by, mix=-RT(XAlnXA + XBlnXB + XCln XC) = -8.314298 (0.78 (ln…
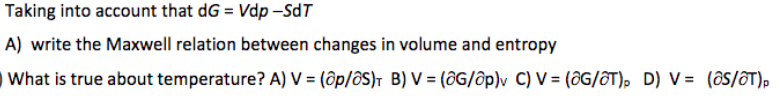
Step by step
Solved in 2 steps with 1 images

- The molar enthalpy of fusion of ice at 273.15 K and one atm is ΔfusHm (H2O)=6.01 kJ mol-1, andthe molar entropy of fusion under the same conditions is ΔfusSm (H2O)=22.0 J K-1 mol-1. Show that(a) ΔfusGm (H2O)=0 at 273.15 K and one atm, (b) ΔfusG,m (H2O) < 0 when the temperature is greaterthan 273.15 K, and (c) ΔfusGm (H2O) > 0 when the temperature is less than 273.15 K.Without carrying out an explicit calculation, explain there lative values of the standard molar entropies (at 298 K) of the following substances: (a) Ne(g) (146 J K-1 mol-1) compared with Xe(g) (170 J K-1 mol-1), (b) H2O(g) (189 J K-1 mol-1) compared with D2O(g) (198 J K-1 mol-1), (c) C(diamond) (2.4 J K-1 mol-1) compared with C(g raphite) (5.7 J K-1 mol-1).The standard Gibbs energy of formation of rhombic sulfur is zero, and that of monoclinic sulfur is +0.33 kJ mol−1 at 25 °C. The standard molar entropy of rhombic sulfur is 31.80 J K−1 mol−1, and that of monoclinic sulfur is 32.6 J K−1 mol−1. At what temperature will the transition occur at 1 bar? _______ K. 3 sig. fig.
- The enthalpy of vaporization (deltaHvap) of water was experimentally determined to be 151.3 kJ for 3.72 mols of water at 100 degrees Celsius? Calculate the molar entropy of vaporaization (deltaSvap,m) of water.Derive a formula for the molar Gibbs free energy change of a closed system at constant temperature if the system obeys the equation of state PV = nRT + nCP where C is a constant.Suppose a certain small bird has a mass of 30 g. What is the minimum mass of glucose that it must consume to fly to a branch 10 m above the ground? The change in Gibbs energy that accompanies the oxidation of 1.0 mol C6H12O6(s) to carbon dioxide and water vapour at 25 °C is -2828 kJ.
- Given a thermodynamic function h=h(s,p) show how to evaluate T, V , U , A or F and GCalculate the molar entropy of an ensemble by S=U/T+klnQ with 1.32 mole of Kr(g) at 47degree and a pressure of 1.7 bar.The change in Gibbs energy that accompanies the combustion of C6H12O6(s) to carbon dioxide and water vapour at 25 °c is -2828 kJ mol-1. The potential energy of an object of mass m at a height h, relative to that at the Earth's surface is given by mgh, where g = 9.81 m s-2 is the acceleration of freefall. How much glucose does a person of mass 65 kg need to consume to climb through 10m?
- a) Find the entropy change associated with melting pure ice at T=273.15K, P=20 bar. b) What is the Gibbs energy of melting pure ice at T=0 C and P=1 bar? c) What is the change in total entropy (system + surroundings) for the melting of pure ice at T=0 C and P=1 bar?The temperature dependence of the heat capacity of non-metallic solids is found to follow the Debye T3 -law at very low temperatures, with Cp,m = aT3. (a) Derive an expression for the change in molar entropy on heating for such a solid. (b) For solid nitrogen, a= 6.15 x 10-3 J K-4 mol-1. What is the molar entropy of solid nitrogen at 5 K?What would the entropy be for a system with 6.05E+1 microstates, in J/mol-K?

