tes > SL 2 Resources Determine the overall charge on particles with the subatomic makeups given. 13 protons, 14 neutrons, and 10 electrons 8 protons, 10 neutrons, and 8 electrons 15 protons, 18 neutrons, and 18 electrons 3 protons, 3 neutrons, and 2 electrons privscy policy sbout us terms of usE contact us help Careers
tes > SL 2 Resources Determine the overall charge on particles with the subatomic makeups given. 13 protons, 14 neutrons, and 10 electrons 8 protons, 10 neutrons, and 8 electrons 15 protons, 18 neutrons, and 18 electrons 3 protons, 3 neutrons, and 2 electrons privscy policy sbout us terms of usE contact us help Careers
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter21: Nuclear Chemistry
Section: Chapter Questions
Problem 4E: For each of the isotopes in Exercise 21.1, determine the numbers of protons, neutrons, and electrons...
Related questions
Question
Determine the overall charge on particles with the subatomic makeup given.
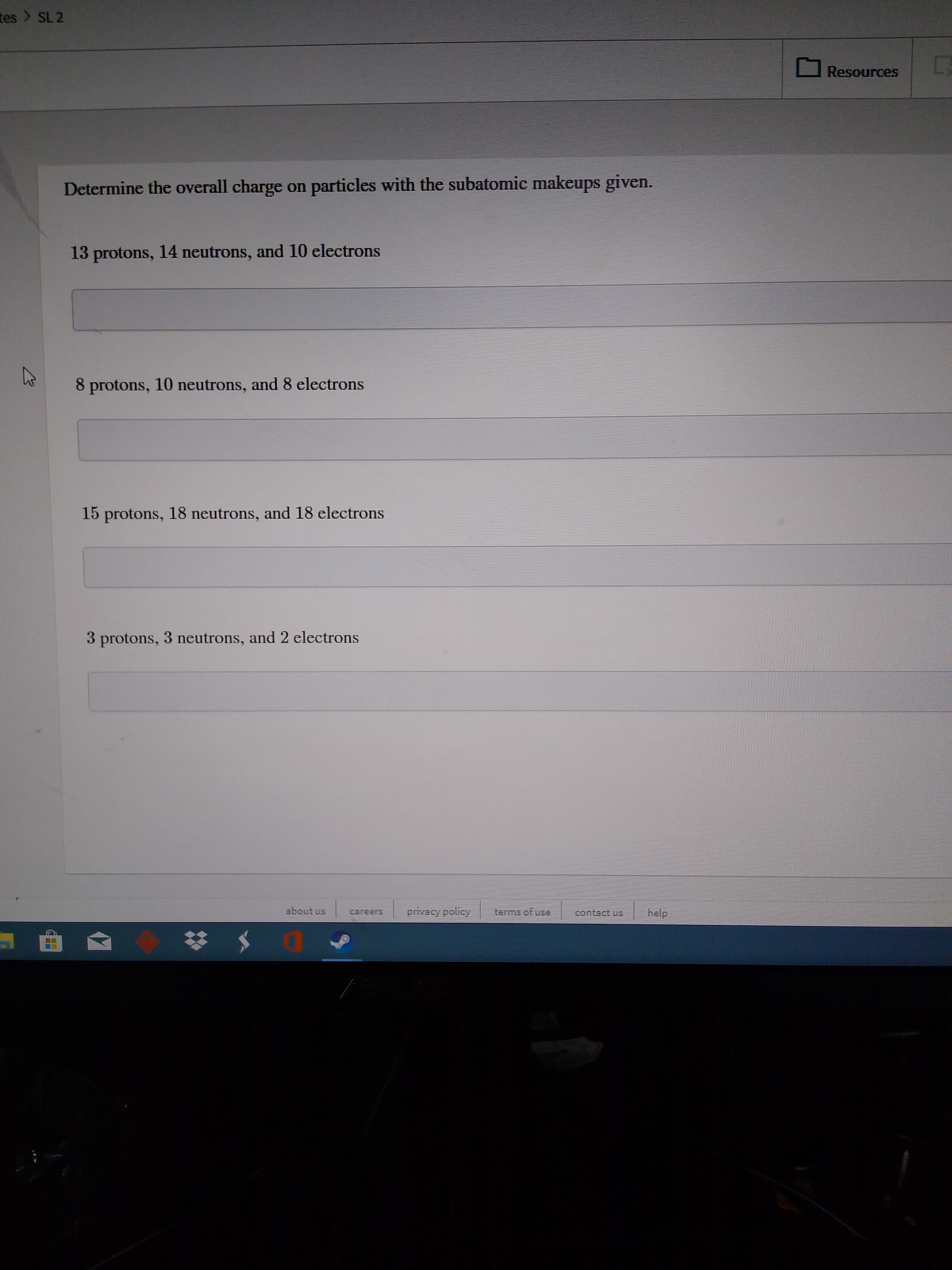
Transcribed Image Text:tes > SL 2
Resources
Determine the overall charge on particles with the subatomic makeups given.
13 protons, 14 neutrons, and 10 electrons
8 protons, 10 neutrons, and 8 electrons
15 protons, 18 neutrons, and 18 electrons
3 protons, 3 neutrons, and 2 electrons
privscy policy
sbout us
terms of usE
contact us
help
Careers
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning