The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108 kJ/mol. If an enzyme increases the rate of the hydrolysis by a factor of (7.07x10^0)E6, how much is the activation energy of the catalyzed reaction? Assume the frequency (A) factors for the catalyzed and the uncatalyzed reactions are identical and the temperature is 25 deg. C.
The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108 kJ/mol. If an enzyme increases the rate of the hydrolysis by a factor of (7.07x10^0)E6, how much is the activation energy of the catalyzed reaction? Assume the frequency (A) factors for the catalyzed and the uncatalyzed reactions are identical and the temperature is 25 deg. C.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 75E: One mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a...
Related questions
Question
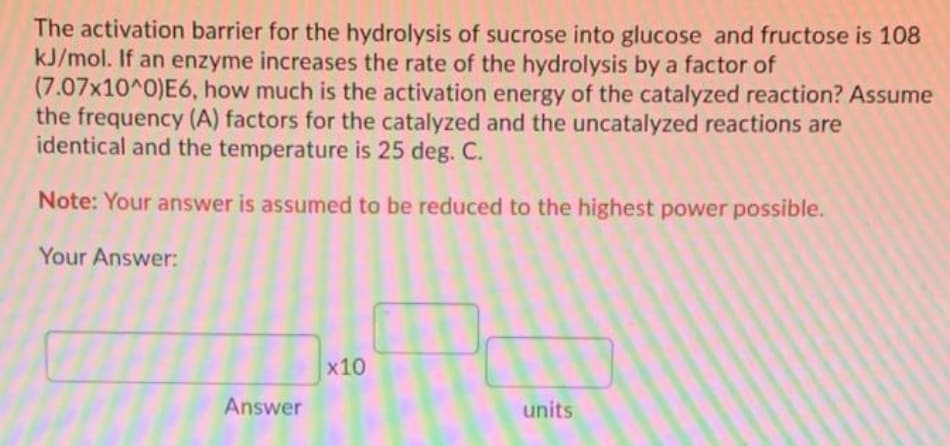
Transcribed Image Text:The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108
kJ/mol. If an enzyme increases the rate of the hydrolysis by a factor of
(7.07x10^0)E6, how much is the activation energy of the catalyzed reaction? Assume
the frequency (A) factors for the catalyzed and the uncatalyzed reactions are
identical and the temperature is 25 deg. C.
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
x10
Answer
units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning