The amino acid glycine, CH5NO2, is one of the compounds used by the body to make proteins. The equation for its combustion is 4C2H5NO2(s) + 902(g) → 8CO;(g) +1OH;0(1) +2N,(3) For each mole of glycine that burns, 973.49 kJ of heat is liberated. Use this information, plus values of AH° for the products of combustion, to calculate AH° for glycine. AH;° = i ! kJ/mol
The amino acid glycine, CH5NO2, is one of the compounds used by the body to make proteins. The equation for its combustion is 4C2H5NO2(s) + 902(g) → 8CO;(g) +1OH;0(1) +2N,(3) For each mole of glycine that burns, 973.49 kJ of heat is liberated. Use this information, plus values of AH° for the products of combustion, to calculate AH° for glycine. AH;° = i ! kJ/mol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 105AE: Combustion of table sugar produces CO2(g) and H2O( l). When 1.46 g table sugar is combusted in a...
Related questions
Question

Transcribed Image Text:The amino acid glycine, CH5NO2, is one of the compounds used by the body to make proteins. The equation for its combustion is
4C2H5NO2(s) + 902(g) → 8CO;(g) +1OH;O(1) +2N,(g)
For each mole of glycine that burns, 973.49 kJ of heat is liberated. Use this information, plus values of AH° for the products of
combustion, to calculate AH;° for glycine.
AH;° = i
! kJ/mol
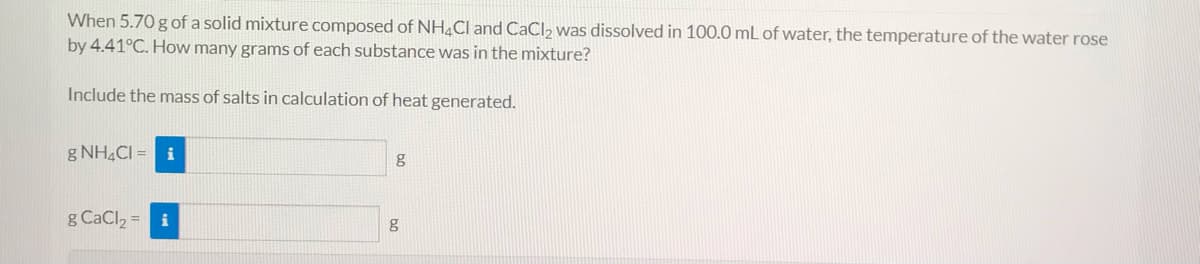
Transcribed Image Text:When 5.70 g of a solid mixture composed of NH4CI and CaCl2 was dissolved in 100.0 mL of water, the temperature of the water rose
by 4.41°C. How many grams of each substance was in the mixture?
Include the mass of salts in calculation of heat generated.
g NH4CI = i
g
g CaCl2 = i
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning