The balanced reaction for the combustion of ethane is shown in the table You allow 25 mol of ethane (C, Ha) to react with 3.4 mol oxygen (O,) Complete the following ICF table, which may be done using the Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions Drag the appropriate amounts to their respective targets.
The balanced reaction for the combustion of ethane is shown in the table You allow 25 mol of ethane (C, Ha) to react with 3.4 mol oxygen (O,) Complete the following ICF table, which may be done using the Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions Drag the appropriate amounts to their respective targets.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
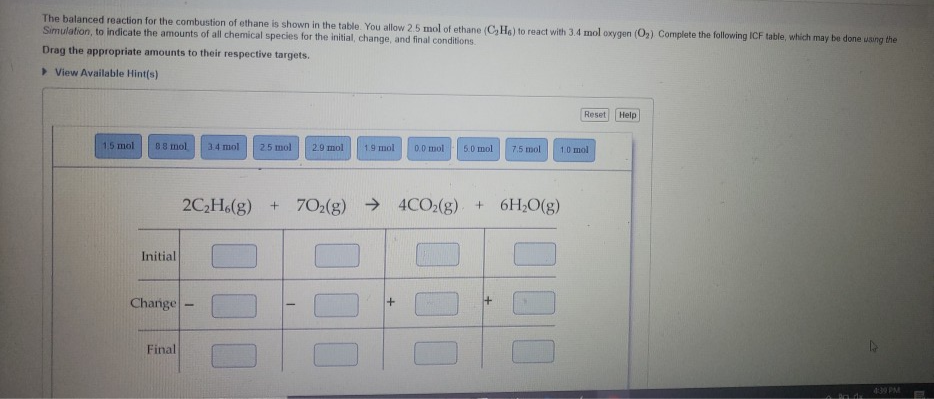
Transcribed Image Text:The balanced reaction for the combustion of ethane is shown in the table. You allow 2.5 mol of ethane (C, He) to react with 3.4 mol oxygen (O,) Complete the following ICF table, which may be done using the
Simulation, to indicate the amounts of all chemical species for the initial, change, and final conditions.
Drag the appropriate amounts to their respective targets.
> View Available Hint(s)
Reset
Help
1.5 mol
88 mol
34 mol
2.5 mol
2.9 mol
1.9 mol
0.0 mol
5.0 mol
7.5 mol
1.0 mol
2C,H6(g) + 702(g) →
4CO2(g) + 6H;O(g)
Initial
Change
Final
4:39 PM
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning