The combustion of 1.793 g of fructose, C,H,0,(s), in a bomb calorimeter with a heat capacity of 5.30 kJ/°C results in an increase in the temperature of the calorimeter and its contents from 22.25 °C to 27.53 °C. What is the internal energy change, AU, for the combustion of 1.793 g of fructose? AU = kJ Calculate the enthalpy of combustion, AHc, of fructose in kilojoules per mole. ΔΗ, kJ/mol
The combustion of 1.793 g of fructose, C,H,0,(s), in a bomb calorimeter with a heat capacity of 5.30 kJ/°C results in an increase in the temperature of the calorimeter and its contents from 22.25 °C to 27.53 °C. What is the internal energy change, AU, for the combustion of 1.793 g of fructose? AU = kJ Calculate the enthalpy of combustion, AHc, of fructose in kilojoules per mole. ΔΗ, kJ/mol
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 30E: When 1.0 g of fructose, C6H12O6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Related questions
Question
What is the internal energy change, Δ?ΔU, for the combustion of 1.7931.793 g of fructose?
Calculate the enthalpy of combustion, Δ?cΔHc, of fructose in kilojoules per mole.
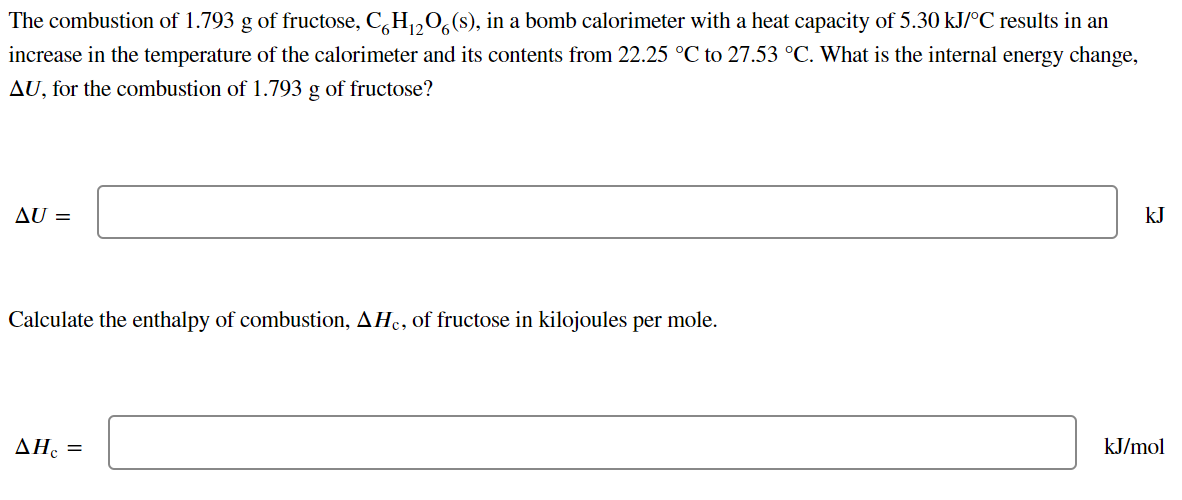
Transcribed Image Text:The combustion of 1.793 g of fructose, C,H,,0, (s), in a bomb calorimeter with a heat capacity of 5.30 kJ/°C results in an
increase in the temperature of the calorimeter and its contents from 22.25 °C to 27.53 °C. What is the internal energy change,
AU, for the combustion of 1.793 g of fructose?
AU =
kJ
Calculate the enthalpy of combustion, AHc, of fructose in kilojoules per mole.
ΔΗ, -
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
