The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: → 4 NO2 (g) + 6 H2O (g) 4 NH3 (g) +7 02 (g) The combustion of 175 g of ammonia consumes g of oxygen. Atomic weights: N=14.01, H=1.01, O=15.99. Input values only with 2 decimal places.
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: → 4 NO2 (g) + 6 H2O (g) 4 NH3 (g) +7 02 (g) The combustion of 175 g of ammonia consumes g of oxygen. Atomic weights: N=14.01, H=1.01, O=15.99. Input values only with 2 decimal places.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 100QRT: Using Table 1.1, but without using your calculator, decide which has the larger mass:
20. mL butane...
Related questions
Question
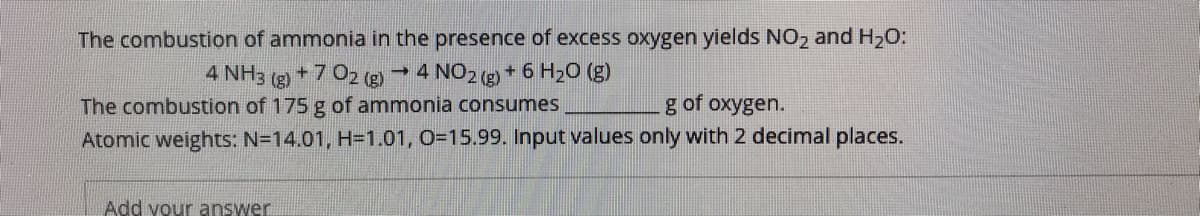
Transcribed Image Text:The combustion of ammonia in the presence of excess oxygen yields NO, and H20:
4 NH3 (g) +7 02 (g) 4 NO2 (g) + 6 H20 (g)
The combustion of 175 g of ammonia consumes
g of oxygen.
Atomic weights: N=14.01, H=1.01, O=15.99. Input values only with 2 decimal places.
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning