![The complex (Fe(H,O)]* is paramagnetic. Is the H,0 ligand
inducing a strong or weak field?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7205430a-85db-41e8-8e3e-d36cca36d5fa%2Fe94943c5-7f4d-4996-b23e-ba8621412f24%2Fwhcbeh.png&w=3840&q=75)
The coordination complex is the compound that does not lose its identity in the solution. The coordination complex must have a central metal atom and ligand that are bonded together by a coordinate covalent bond. The complex is said to be paramagnetic if it has unpaired electrons in its orbitals while if it has paired electrons it is said to be diamagnetic. A strong field ligand helps in the pairing of electrons while the weak field lgand can not pair the electrons.
To determine whether water molecule acts as a strong or weak field ligand.
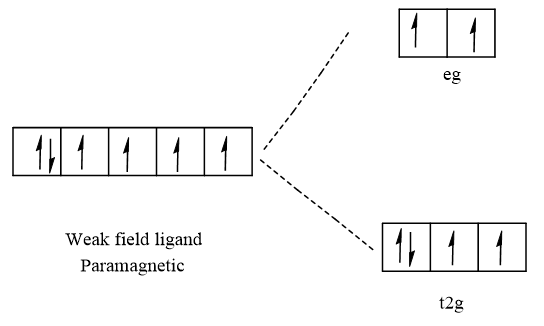
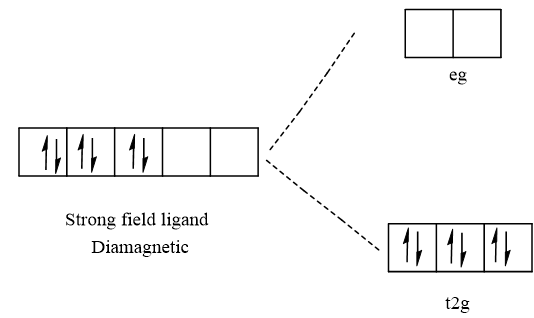
The given complex is [Fe(H2O)6]2+ is paramagnetic in nature. The central atom Fe has the atomic number 26 and it's ground state electronic configuration is [Ar]3d64s2. In the given complex Fe has oxidation number +2 that means it loses two-electron from 4s orbitals. So, the electronic configuration of Fe2+ is [Ar]3d6. The filling of electrons in the octahedral complex of Fe2+ in the case of strong and weak field ligands. The electronic configuration in case of weak field ligand is t2g4eg2. The electronic configuration in the case of a strong field ligand is t2g6eg0. Hence, the given complex is paramagnetic due to the presence of unpaired electrons in the case of a weak field ligand. Therefore, water is a weak field ligand.
Weak field ligand
Strong filed ligand
Step by step
Solved in 3 steps with 2 images









