The compound 1,3-di-t-butylcyclohexane exists in two forms that are known as the chair and boat conformations because their molecular structures resemble those objects. Equilibrium exists between the two forms, represented by the equation chair boat At 580 K, 6.42% of the molecules are in the chair form. Calculate the equilibrium constant for the preceding reac- tion as written.
The compound 1,3-di-t-butylcyclohexane exists in two forms that are known as the chair and boat conformations because their molecular structures resemble those objects. Equilibrium exists between the two forms, represented by the equation chair boat At 580 K, 6.42% of the molecules are in the chair form. Calculate the equilibrium constant for the preceding reac- tion as written.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 23P
Related questions
Question
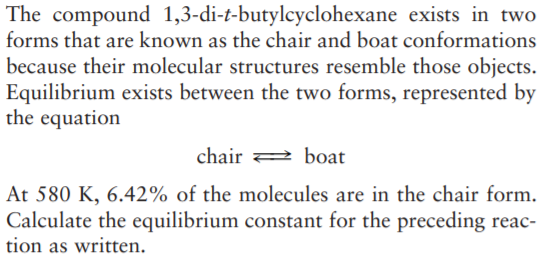
Transcribed Image Text:The compound 1,3-di-t-butylcyclohexane exists in two
forms that are known as the chair and boat conformations
because their molecular structures resemble those objects.
Equilibrium exists between the two forms, represented by
the equation
chair
boat
At 580 K, 6.42% of the molecules are in the chair form.
Calculate the equilibrium constant for the preceding reac-
tion as written.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning